- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Induction, Isolation and Counting of Akinetes in Aphanizomenon ovalisporum
Published: Vol 6, Iss 10, May 20, 2016 DOI: 10.21769/BioProtoc.1808 Views: 8513
Reviewed by: Maria SinetovaClaudia CatalanottiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation of Commensal Escherichia coli Strains from Feces of Healthy Laboratory Mice or Rats
Tingting Ju and Benjamin P. Willing
Mar 20, 2018 10915 Views

Detachment Procedure of Bacteria from Atmospheric Particles for Flow-cytometry Counting
Carolina M. Araya [...] Isabel Reche
Jun 20, 2019 5593 Views

Shipment of Cyanobacteria by Agarose Gel Embedding (SCAGE)—A Novel Method for Simple and Robust Delivery of Cyanobacteria
Phillipp Fink [...] Karl Forchhammer
Dec 5, 2024 1399 Views
Abstract
Akinetes are spore-like resting (dormant) cells formed by strains of filamentous cyanobacteria for surviving long periods of unfavorable conditions. During deprivation for potassium, vegetative photosynthetic cells along the filaments of the cyanobacterium Aphanizomenon ovalisporum (A. ovalisporum) (strain ILC-164) differentiate into akinetes. Akinetes are larger than vegetative cell, have a thick wall, accumulate storage compounds (cyanophycine, glycogen, lipids) and excess of DNA (Sukenik et al., 2015; Sukenik et al., 2007; Maldener et al., 2014). Differences in structure and composition between akinetes and vegetative cells allow separation and isolation of akinetes. Akinetes isolated by the described protocol can be utilized for protein analysis, measurements of metabolic activities, fluorescence in situ hybridization (FISH) studies and more.
Materials and Reagents
- Erlenmeyer flasks of appropriate volume
- 50 ml tubes (SARSTEDT AG & Co, catalog number: 62.547.004 )
- 250 ml centrifuge bottle (TermoFisher SCIENTIFIC, catalog number: 3141-0250 )
- Aphanizomenon ovalisporum, strain ILC-164 [isolated from Lake Kinneret, Israel (Banker et al., 1997)]
- BG11 growth medium (Stanier et al., 1971)
- BG/-K akinete induction medium
Note: BG11 medium in which the K2HPO4 component was substituted with Na2HPO4. - Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- Ethylenediaminetetraacetic acid disodium salt dehydrate (Na-EDTA) (Sigma-Aldrich, catalog number: E5134 )
- Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266 )
- Lysozyme from chicken egg white (Sigma-Aldrich, catalog number: L6876 )
Note: It is also named as “Mucopeptide N-acetylmuramoylhydrolase” or “muramidase”. - Lugol’s solution (Sigma-Aldrich, catalog number: L6146 )
- SYTOX (Invitrogen, catalog number: S7020 )
Note: Currently, it is “SYTOX® Green Nucleic Acid Stain-5 mM Solution in DMSO” (Thermo Fisher Scientific, Molecular ProbesTM, catalog number: S7020). - Coomassie brilliant blue R-250 staining solution (BIO RAD catalog number: 1610436 ).
- Molecular weight markers [All blue pre-stained protein standards (M. W. 10-250 kD)] (Bio-Rad Laboratories, catalog number: 1610373 )
- Tris (Sigma-Aldrich, catalog number: 252859 )
Note: It is also named as “Tris (hydroxymethyl) aminomethane” on Sigma-Aldrich website. - Tris buffer(see Recipes)
- Tris-EDTA-Mg buffer (see Recipes)
- Tris-Mg buffer (see Recipes)
- TE buffer (see Recipes)
- 0.5 M EDTA stock solution (see Recipes)
- 1 M MgCl2 stock solute (see Recipes)
- 1 M NaCl (see Recipes)
Equipment
- Spectrophotometer (such as Uvikon XS SECOMAM)
- Sorvall centrifuge RC 6 Plus and appropriate rotor for the centrifuge tubes or bottles
- Vortex
- Sonicator [e.g., Sonifier 450 (Branson Ultrasonics)]
- Orbital shaker (e.g., MaxQTM 2000 and 3000 benchtop orbital shaker, TermoFisher SCIENTIFIC, model number: SHKA2000 )
- Incubator (regulated temperature and light)
- Bright field/fluorescent inverted microscope (e.g., Zeiss Axioobserver Z1)
Note: Use the following filter sets: for chlorophyll (EX-425-443 nm; BS-452 nm; EM-496 nm LP), for Phycobilins (EX-510-550 nm; BS-565 nm; EM-582 nm LP) and for SYTOX (EX-445-495 nm; BS-500 nm; EM-505-555 nm). - Utermohl sedimentation chamber (Aquatic Research Instruments, http://www.aquaticresearch.com/sedimentation_chamber.htm)
- Thermostatic water bath
Procedure
- Cultivation and akinete induction
- In order to obtained sufficient biomass for akinete induction, cultivate A. ovalisporum in Erlenmeyer flasks using 0.5-1.0 liters BG11 medium. Incubate cultures at 28 ℃ under continuous light (PAR) at intensity of 30 µmol photon m-2 s-1. Culture can be aerated at 0.5 liter/min using aquarium air pump.
- Toward the end of the exponential growth phase (after 7-10 days, OD750 should be around 1.0. In case this OD has not reach consider restart this step with a new inoculum), remove the culture to a lower temperature incubator (20-24 ℃). Allow 3-4 days of acclimation under continuous light (PAR) at intensity of 30 µmol photon m-2 s-1 and aeration. Then harvest the culture by centrifugation.
- Centrifuge the culture at 5,000 x g for 5-10 min at room temperature, discard the supernatant. If available, use a centrifuge with the rotor fitting 500 ml tubes or distribute the culture into 50 ml Falcon tubes and perform several rounds of centrifugation. At the end of centrifugation collect the biomass in one 50 ml falcon tube and discard as much of growth medium as possible.
- Wash the pellet in BG/-K medium 3 times by re-suspending the pellet in the potassium depleted medium by gently vortexing, followed by centrifugation at 5,000 x g at room temperature. Finally re-suspend the pellet in 0.5 liters BG/-K medium using a 1-liter Erlenmeyer flask. Incubate the K deprived culture at a controlled temperature between 20 and 24 ℃ under continuous light (PAR) at intensity of 70-80 µmol photon m-2 s-1.
- Sample 3-5 ml from the induced culture to follow the progress of the akinete differentiation process. Collect samples at days 4, 7, 10, 14, 21 and 28-post induction. Young akinetes attached to filaments can be observed since day 5 or 7. Later on, more attached akinetes can be observed together with free akinetes whose diameter is larger than the young attached akinetes (Figure 1).
Note: Akinete differentiation is a non-synchronized process.
- In order to obtained sufficient biomass for akinete induction, cultivate A. ovalisporum in Erlenmeyer flasks using 0.5-1.0 liters BG11 medium. Incubate cultures at 28 ℃ under continuous light (PAR) at intensity of 30 µmol photon m-2 s-1. Culture can be aerated at 0.5 liter/min using aquarium air pump.
- Isolation of akinete [modified from Razquin et al. (1996)]
- Centrifuge a 21 (or 28) days old akinete-induced culture at 5,000 x g at room temperature for 5-10 min, discard the supernatant.
- Re-suspend the pellet and wash few times in Tris-EDTA-Mg buffer. Finally re-suspend the washed pellet in 20-50 ml Tris-Mg buffer and add lysozyme to a final concentration of 1 mg ml-1. Use freshly prepared lysozyme stock solution.
- Incubate the suspension for 2 h at 37 ℃ to allow the disruption of vegetative cells, followed by low speed (3,000 x g) centrifugation for 3 min. During the incubation period, sample the suspension for microscopic observation of cell disruption. If necessary, extend the incubation with lysozyme for an additional hour.
- Re-suspend the akinete-enriched pellet in TE buffer and wash several times in the same buffer to remove cellular residues and the soluble lysozyme.
- Finally re-suspend the akinete enriched pellet in Tris buffer keep on ice, and briefly sonicate it using 30% of the full intensity at 20 sec intervals (Sonifier 450). Repeat the sonication steps 3-5 times but monitor the akinete integrity under microscope. The aim of this sonication step is to disrupt intact vegetative cells that remained in the suspension. Be sure that the sonication is not too aggressive and akinetes survive this step.
- Centrifuge the akinete suspension at low speed (3,000 x g), wash it a few more times in sterile distilled water and finally re-suspend the pellet in a small volume of sterile distilled water. Keep the suspension at 15 ℃ and dark conditions. An akinetes’ suspension can be kept under those conditions for at least 6 months.
- At this stage, sample an aliquot from the akinete suspension for microscopic observation and enumeration.
- Centrifuge a 21 (or 28) days old akinete-induced culture at 5,000 x g at room temperature for 5-10 min, discard the supernatant.
- Counting akinetes
- Collect aliquots (1-3 ml) from cultures at various stages of akinete differentiation or during the akinete isolation process. Collect samples after vigorous shaking of the culture to ensure homogeneous suspension of filaments and akinetes.
- Preserve the samples in Lugol’s solution (add 1 drop-50 µl to 3 ml sample). Lugol-preserved samples can be stored at room temperature under dark until counting. Preserved samples can be stored for 4-6 months.
- Filaments, vegetative cells and akinetes are counted in an Utermohl sedimentation chamber using an inverted microscope (Utermohl, 1958). Two categories of akinetes can be defined; filament-attached akinetes (Figure 1. D7 and D14) and mature free akinetes (Figure 1. D21 and IA). The size of filament-attached akinetes increased as their differentiation proceeded and they exceeded a biomass of 0.4 ng (Sukenik et al., 2013). During their differentiation, akinetes are characterized by a thickened the cell wall and the accumulation of apparent cyanophycin granules (Figure 1).
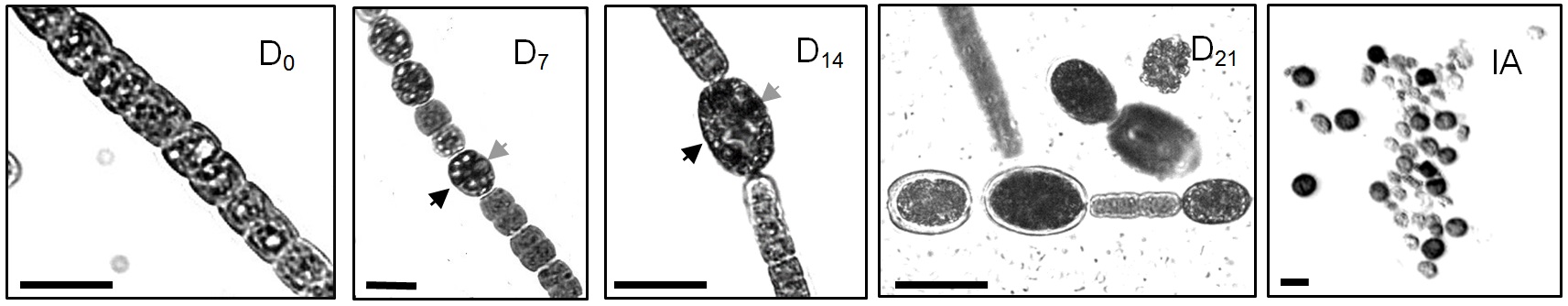
Figure 1. Light micrographs of filaments and akinetes from akinete induced culture of Aphanizomenon ovalisporum. D0, A filament with vegetative cells prior the induction (transfer to a BG/-K medium); D7, A filament 7 days post induction. Note the presence of young akinetes (arrowhead); D14, A filament 14 days post induction. A well-developed attached akinetes is marked with an arrowhead; D21, A short filament with attached akinetes and free akinetes 21 days post induction; IA, Isolated akinetes. Gray arrowheads in D7 and D14 indicate cyanophycine granule. Scale bar = 10 µm
- Collect aliquots (1-3 ml) from cultures at various stages of akinete differentiation or during the akinete isolation process. Collect samples after vigorous shaking of the culture to ensure homogeneous suspension of filaments and akinetes.
- Quality control of isolated akinetes
- Residual lysozyme on isolated akinetes
Although the isolated akinetes suspension is vigorously washed to remove excess lysozyme, we found residual lysozyme in the akinete fraction, presumably associated with cell walls. These residues may interfere later on with akinete germination. The removal of the enzyme from the isolated akinetes is carried out by several washes with TE Buffer containing 100 mM NaCl as assessed by SDS-12% PAGE (Figure 2).
Note: Three washes with TE + 100 mM NaCl are sufficient to remove residual lysozyme from lysozyme treated isolated akinetes. - Assessment of akinete vitality
In order to assess the percentage of vital akinetes use SYTOX solution at final concentration of 1 µM. SYTOX is a high affinity nucleic acid stain that can enter the cell only through damaged plasma membranes, such as those of dead cells. Thus it is possible to discriminate between dead and live akinetes. As a confirmation of vital akinetes, it is recommended to use SYTOX staining in addition to auto-fluorescence of the phycobilisome antenna and Chlorophyll a in viable akinetes (Figure 3).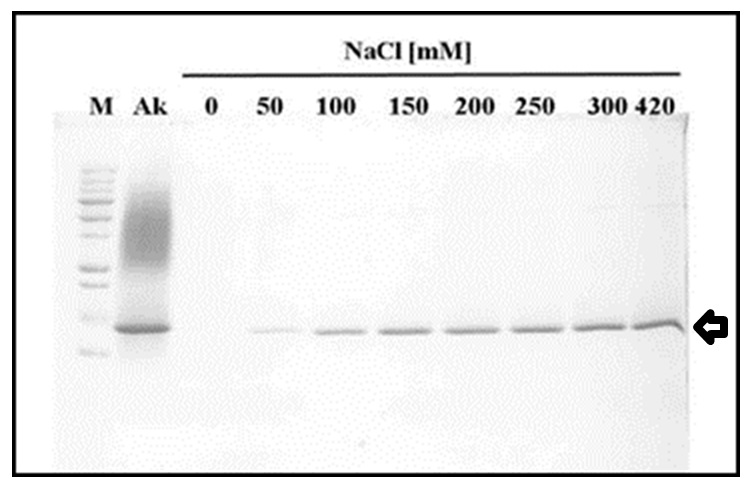
Figure 2. The effect of salt titration on lysozyme dissociation from akinetes. Proteins were separated on Laemmmli SDS-PAGE system (12% acrylamide) and stained by Coomassie blue. M, Molecular Weight Markers (All Blue pre-stained protein standards, M. W. 10-250 kD); AK, crude soluble protein extracted from isolated akinetes; Lanes 0 to 420 are soluble proteins after isolated akinete were washed with TE Buffer + NaCl at different concentrations (0-420 mM NaCl, respectively). Lyzozyme peptide (MW 14.4 kD) released by that treatment is indicated by an arrow. Notice the higher the salt concentration more lysozyme dissociated from isolated akinetes.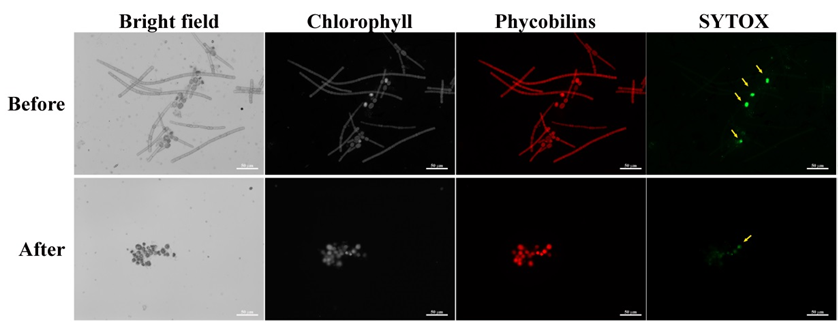
Figure 3. Akinete induced Aphanizomenon ovalisporum samples before and after isolation of akinetes. Upper panel, culture at day 17 of induction; Lower panel, Isolated akinetes. Samples were stained with SYTOX and observed using fluorescence microscope with filters’ cubes set for fluorescence of chlorophyll (EX- 425-443 nm; BS-452 nm; EM-496 nm LP), Phycobilins (EX-510-550 nm; BS-565 nm; EM-582 nm LP) and SYTOX (EX-445-495 nm; BS-500 nm; EM-505-555 nm). Notice the four dead (SYTOX positive) akinetes prior to isolation (yellow arrows) and one dead akinete post isolation. Scale bar = 50 µm
- Residual lysozyme on isolated akinetes
Notes
- Notes about reproducibility and variability:
- Optimal conditions for akinete induction are potassium deficiency, medium light intensity (75 µmol photon m-2 s-1) and temperature of 20-24 ℃ as described by Sukenik et al. (2013).
- Follow the differentiation process every 3-4 days as some variation may occur in the development schedule.
- Optimal conditions for akinete induction are potassium deficiency, medium light intensity (75 µmol photon m-2 s-1) and temperature of 20-24 ℃ as described by Sukenik et al. (2013).
- Other notes and technical tips:
- The final concentration of lysozyme and the incubation time can be slightly adjusted as you gain experience with this protocol.
- Gentle sonication is required at step 5B of the isolation procedure. Follow the effectivity of the sonication in between cycles by microscopic observation.
- The final concentration of lysozyme and the incubation time can be slightly adjusted as you gain experience with this protocol.
Recipes
- Tris buffer
50 mM Tris
pH 7.5 - Tris-EDTA-Mg buffer
50 mM Tris (pH 7.5) containing 10 mM EDTA and 1 mM MgCl2 - Tris-Mg buffer
50 mM Tris (pH 7.5) containing 1 mM MgCl2 - TE buffer
50 mM Tris (pH 7.5) with 10 mM EDTA - 0.5 M EDTA stock solution
Na-EDTA (186.12 g/L) - 1 M MgCl2 stock solute (95.21 g/L)
- 1 M NaCl (58.44 g/L)
Acknowledgments
The protocol was modified from Razquin et al. (1996). The work was supported by research grants from the Israel Science Foundation (ISF grant No. 319/12), a Joint German-Israeli-Project (FKZ 02WT0985, WR803) from German Ministry of Research and Technology (BMBF) and Israel Ministry of Science and Technology (MOST). R. N. K-L was supported by Levy Eshkol Fellowship (No. 3-9476) Israel Ministry of Science and Technology (MOST).
References
- Banker, R., Carmeli, S., Hadas, O., Teltsch, B., Porat, R. and Sukenik, A. (1997). Identification of cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophyceae) isolated from Lake Kinneret, Israel. Journal of Phycology 33(4): 613-616.
- Maldener, I., Summers, M. L. and Sukenik, A. (2014). Cellular differentiation in Filamentous Cyanobacteria. In: Flores, E. and Herrero, A. (eds). The cell biology of cyanobacteria. Caister Academic Press: 263-290.
- Razquin, P., Fillat, M. F., Schmitz, S., Stricker, O., Bohme, H., Gomez-Moreno, C. and Peleato, M. L. (1996). Expression of ferredoxin-NADP+ reductase in heterocysts from Anabaena sp. Biochem J 316 (Pt 1): 157-160.
- Stanier, R. Y., Kunisawa, R., Mandel, M. and Cohen-Bazire, G. (1971). Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35(2): 171-205.
- Sukenik, A., Kaplan-Levy, R. N., Viner-Mozzini, Y., Quesada, A. and Hadas, O. (2013). Potassium deficiency triggers the development of dormant cells (akinetes) in Aphanizomenon ovalisporum (Nostocales, Cyanoprokaryota)(1). J Phycol 49(3): 580-587.
- Sukenik, A., Maldener, I., Delhaye, T., Viner-Mozzini, Y., Sela, D. and Bormans, M. (2015). Carbon assimilation and accumulation of cyanophycin during the development of dormant cells (akinetes) in the cyanobacterium Aphanizomenon ovalisporum. Front Microbiol 6: 1067.
- Sukenik, A., Beardall, J. and Hadas, O. (2007). Photosynthetic characterization of developing and mature akinetes of Aphanizomenon ovalisporum (Cyanoprokaryota). Journal of Phycology 43(4): 780-788.
- Utermohl, H. (1958). Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Inter Ver Theor Ange Limnol 9:1-38.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sukenik, A., Kaplan-Levi, R. N., Viner-Mozzini, Y., Lupu, A. and Sela, D. (2016). Induction, Isolation and Counting of Akinetes in Aphanizomenon ovalisporum. Bio-protocol 6(10): e1808. DOI: 10.21769/BioProtoc.1808.
Category
Microbiology > Microbial cell biology > Cell isolation and culture
Microbiology > Microbial cell biology > Cell viability
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











