- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mating and Progeny Isolation in the Corn Smut Fungus Ustilago maydis
Published: Vol 6, Iss 8, Apr 20, 2016 DOI: 10.21769/BioProtoc.1793 Views: 12064
Reviewed by: Zhaohui LiuChijioke JoshuaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2645 Views

In Vitro Screening of Microbial Extracts Against the Oomycetes Phytophthora capsici and Pythium ultimum
Mónica Trigal Martínez [...] María Ángeles Vinuesa Navarro
Sep 20, 2025 1265 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1582 Views
Abstract
The corn smut pathogen, Ustilago maydis (U. maydis) (DC.) Corda, is a semi-obligate plant pathogenic fungus in the phylum Basidiomycota (Alexopoulos et al., 1996). The fungus can be easily cultured in its haploid yeast phase on common laboratory media. However, to complete its sexual cycle U. maydis strictly requires its specific plant host, maize (Zea mays). The fungus is an interesting and important model organism for the study of the interactions of fungal biotrophic pathogens with plants. In this protocol, we describe the process of plant inoculation, teliospore recovery, germination, progeny isolation and initial mating type analysis. The primary purpose of this protocol is to identify individual progeny strains of U. maydis that can be used for downstream genetic analyses. Generation of targeted mutants to study various processes is a common approach with this and many plant pathogenic fungi. The ability to generate combinations of mutations is facilitated by sexual crossing without the need for additional selectable markers.
Materials and Reagents
- Plastic sterile syringes: 3 ml (BD, catalog number: 309657 ) and 10 ml (BD, catalog number: 309604 )
- Needles: 22 gauge (BD, EclipseTM, catalog number: 305768 ) and 27 gauge (BD, EclipseTM, catalog number: 305758 )
- Conical tubes: Falcon® 50 ml (Corning, catalog number: 352070 )
- Test tubes
- 1.5 ml Microfuge tubes (Thermo Fisher Scientific, catalog number: 05-408-130 )
- Haploid Ustilago maydis tester strains of known mating type
- Seeds for maize seedling assay: Variety Golden Bantam (Rich Farm Garden Supply)
- Seeds for maize ear inoculation: Variety Tom Thumb (Seed Savers Exchange)
- Sterile distilled water (sdH2O)
- CuSO4 solution (1%) (Sigma-Aldrich, catalog number: 451657 )
- Casamino acids (Becton, Dickinson and Company, catalog number: 223050 )
- Ammonium nitrate (Sigma-Aldrich, catalog number: A9642 )
- Yeast extract (Sigma-Aldrich, catalog number: Y1625 )
- Activated charcoal (Sigma-Aldrich, catalog number: C9157 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655 )
- Sodium sulfate (Na2SO4) (Sigma-Aldrich, catalog number: 239313 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: 223506 )
- Boric acid (H3BO3 ) (Sigma-Aldrich, catalog number: B6768 )
- Manganese(II) chloride (MnCl2) (Sigma-Aldrich, catalog number: 244589 )
- Zinc chloride (ZnCl2 ) (Sigma-Aldrich, catalog number: 746355 )
- Sodium molybdate dehydrate (Na2MoO4·2H2O) (Sigma-Aldrich, catalog number: 331058 )
- Iron(III) chloride (FeCl3) (Sigma-Aldrich, catalog number: 157740 )
- Potato dextrose agar (PDA) 2% agar (Sigma-Aldrich) (see Recipes)
- Potato dextrose broth (PDB) (Sigma-Aldrich) (see Recipes)
- Charcoal mating plate medium (see Recipes)
- U. maydis salt solution (Sigma-Aldrich) (see Recipes)
- Trace element solution (Sigma-Aldrich) (see Recipes)
Equipment
- Glass stirring rods
- Pipettes
- Ceramic mortars and pestles
- Sterile cheesecloth
- Hemocytometer or automated cell counter
- Light microscope
- Centrifuge holding 50 ml conical tubes (Beckman Coulter, model: J25I ) with a JS7.5 rotor (Beckman Coulter)
- Growth chamber (Conviron, model: E15 )
- Laminar flow hood
- Rotational Incubator (VWR International, New Brunswick model: 12500KC )
- Stationary incubator (PrecisionTM, model: 815 )
Procedure
U. maydis teliospores can potentially be produced in any above ground, actively growing tissues. However, for research purposes two plant stages will be described, each with its particular advantages. These commonly used stages are:
- Seedlings (7 days old).
Advantages: Seedlings are convenient for speed and for space conservation. - Maturing ears.
Advantages: Ears have the advantage of producing copious numbers of teliospores and, although the plants must be older, the time from inoculation to spore production period is similar to that of seedlings.
Ear inoculation should be performed before pollination occurs; removing tassels may improve gall yield (Snetselaar et al., 2001).
- Plant inoculation for U. maydis teliospore production
- Streak U. maydis compatible mating strains onto fresh potato dextrose agar (PDA) plates (from older PDA plates or glycerol stocks) and incubate at 30 °C until individual colonies appear (Figure 1). Pick a single colony of compatible mating strains into separate test tubes containing 5 ml of potato dextrose broth (PDB) and grow overnight in a rotary shaker set at 250 rpm and 30 °C. Carefully label each tube with the corresponding mating type strain growing in each tube. PDA U. maydis plates can be stored sealed with parafilm and upside down at 4 °C for 2-3 weeks. Re-streak onto fresh PDA plates prior to future use.
- Make 1/100 dilutions of the overnight cultures and estimate cell concentrations using a hemocytometer or automated cell counter. U. maydis cells are cigar-shaped and measure between 2 and 4 μm in width and 20 and 30 μm in length.
- Prepare 50 ml of cell suspension as follows. Dilute and combine each strain to a final concentration of 106 cells/ml using sdH2O. This mixture will be used to inoculate plants and it is important that compatible U. maydis strains are in roughly equal proportions in the cell mixture to achieve optimal teliospore yield. Cell mixtures are stable for inoculations for at least several hours.
- Inoculation of maize plants
- Maize seedling inoculation (7 days old). One effective injection site per seedling should suffice. However, there is frequently a need to move the needle if injection flow is inhibited. Inoculate each seedling with the cell mixture using a 3 ml disposable plastic sterile syringe fitted with a 27 gauge needle. Insert the needle about half the diameter (ca. 2.5 mm) into the pseudo-stem (the internal cavity formed by growing leaves) about 1 cm above the soil line (Figure 2). Inject enough mixture until the liquid can be observed to rise to the top of the area where leaves begin to expand (between 1-2 ml of cell suspension).
- Maize ear inoculation. Grow ‘Tom Thumb’ maize plants in the greenhouse for approximately 40 days or until the ears begin to develop. Before inoculation, cut silks with scissors to top of ear husks and check young ear (by feeling the exterior of the ears to check if grains are present) to ensure that grain filling had not yet taken place. Using a 10 ml hypodermic syringe with a 22 gauge needle inject cell suspension at multiple locations in the ear until liquid is observed to overflow (3-10 ml) from the point where the silks were cut. Injecting liquid into an ear often requires more force than seedlings; move the needle around to find a suitable spot for efficient injections.
- For all inoculated tissues, allow galls to develop and teliospores to form. This step will take about 3-4 weeks for either tissue (Figure 3).
- Harvest fully developed darkly pigmented galls (coloration is from the teliospores).
- Teliospores can be extracted immediately, or alternatively galls can be let to dry in a paper bag at room temperature for later extractions.
- Teliospore isolation
- Grind and sterilize gall tissue [4-5 small or 1 large fresh or dried gall(s)] in a mortar containing 30 ml 1% CuSO4. Watch for the release of black teliospores during grinding. Teliospores cell walls are extremely tough and therefore will not be damaged during the grinding process.
- Filter the macerated tissue through several layers of sterile cheesecloth into a sterile 50 ml conical tube. Incubate flow-through for 30 min at room temperature to allow antibacterial action of CuSO4.
- Pellet teliospores in centrifuge set at 4,000 x g for 5 min.
- Resuspend the pellet in 50 ml sdH2O.
- Repeat steps B3 through B4 once more.
- Resuspend the washed teliospore pellet in 1 ml sdH2O.
- Spread roughly 50-150 μl of the spore suspension onto two PDA plates amended with 50 μg/ml each of streptomycin and ampicillin (PDA-Strep-Amp).
- Incubate overnight at 30 °C.
- When microcolonies are visible (<0.5 mm diameter) under a stereo microscope, add 1 ml of sdH2O to the plate. Slide a sterilized bent glass rod across the plate to suspend microcolony cells. Pipette the sporidial (cell) suspension into a sterile microfuge tube.
- Plate dilutions (10-1, 10-2, 10-3 dilutions work well) onto fresh PDA-Strep-Amp plates.
- Pick progeny colonies onto fresh plates and analyze for the segregation of the traits of interest.
- Mating assay
The plate mating assay is used during isolation of strains from teliospores to establish the mating type of progeny strains (Andrews et al., 2000). U. maydis has a tetrapolar mating system governed by two independently assorting loci: a and b (Bölker et al., 2012). Mating and further filamentous development require strains to differ at both mating type loci. This assay is also used to test the ability of mutant strains to mate.- Pick genetically pure progeny colonies isolated from teliospores (described above) into 5 ml PDB and grow overnight as described above.
- Grow tester U. maydis strains of known mating type (e.g. a1b1; a2b1; a1b2; a2b2).
- Spotting strains onto charcoal plate.
- Tester strains are spotted first. For each tester spot 3-5 μl onto charcoal forming a column (Figure 4a).
- Let inoculum droplets dry (in a laminar flow hood).
- On top of each tester strain, spot 3-5 μl of each of the progeny strains to be tested (as shown in Figure 4a).
- Incubate plates in the dark at room temperature for 1-2 days. Positive mating events are evidenced by white fuzzy growth due to the development of short filaments indicative of the formation of the dikaryon after mating (Figure 4b).
Occasionally, mutants are isolated which lose their potential to complete part or all of the mating process on charcoal plates. In those cases, confrontation (Snetselaar et al., 1996; Gold et al., 1997) and cytoduction assays (Trueheart and Herskowitz, 1992; Mayorga and Gold, 1999) can be used to test the ability of these strains to undergo the first stages of mating response.
Representative data
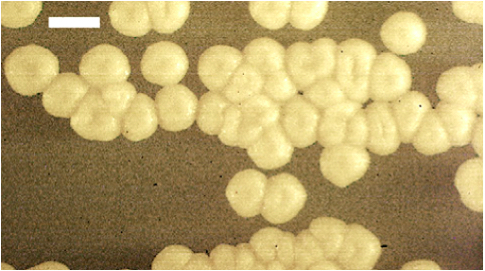
Figure 1. U. maydis haploid colonies. Typical cream colored colonies of U. maydis grown on PDA medium. Size bar is 2 mm.
Figure 2. Inoculation of maize seedlings with a mixture of compatible U. maydis haploid cells. Insert the syringe’s needle half the diameter into the maize seedling pseudo-stem at about 1 cm above the soil line. Inject enough cell mixture to fill the seedling pseudo-stem cavity; observe for liquid expression from top of leaf whorl. 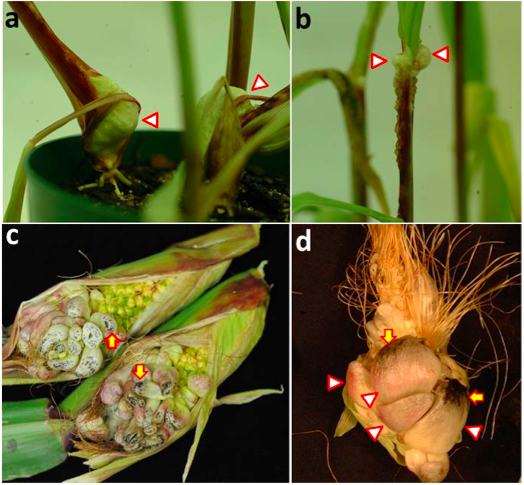
Figure 3. Gall production and teliospore development in maize seedlings and ears inoculated with compatible U. maydis strains. a. Large galls have developed on the lower portion of the seedling stems (arrowheads), close to the soil. b. Series of small galls formed at the distal portion of seedling stem and leaf ligule (arrowhead). c. Young ear galls cut transversely to show teliospore production; note small black areas corresponding to mature teliospores (arrows). d. Mature ear galls; teliospore production increases at later stages of gall development (arrows) and galls become darker in color due to the abundant melanized teliospores. 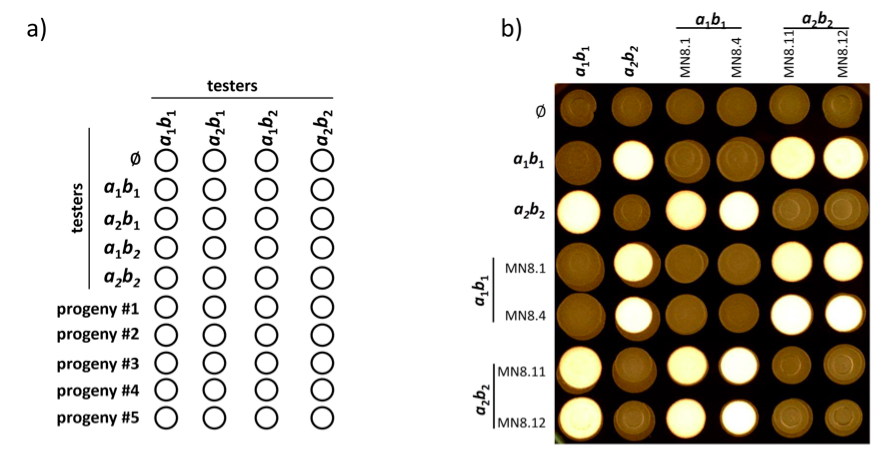
Figure 4. Plate mating assay. a). Representation of suggested mating plate organization. Each isolated strain is co-spotted with tester strains of known mating type to determine mating type of progeny. b). Mating plate results with progeny of previously determined mating type. Four autophagy deletion mutant strains (Nadal and Gold, 2010) (MN8.1; MN8.4; MN8.11 and MN8.12) were tested for their ability to mate. White fuzzy growth indicates positive mating events. ɸ indicates mock (water) inoculation for the corresponding row showing the morphology of unmated strains.
Notes
- Charcoal mating plates should be poured when relatively cool and well mixed in a single layer in direct contact with bench. This is necessary because with prolonged solidification the charcoal will fall out of suspension and reduce the efficiency of the mating reactions.
- For those items without catalog numbers, we have used multiple suppliers with equal success.
- Do not run inoculation experiments in areas where fungicides are sprayed routinely.
Recipes
Note: Chemical reagents were obtained from Sigma-Aldrich.
- Potato dextrose agar (PDA) 2% agar
39 g potato dextrose agar (PDA) powder
5 g supplemental agar
1 L H2O
Autoclave at 121 °C, 15 psi for 15 min - Potato dextrose broth (PDB)
24 g potato dextrose broth powder
1 L H2O
Autoclaved at 121 °C, 15 psi for 15 min - Charcoal mating plate medium
10 g casamino acids
3 g ammonium nitrate
20 g yeast extract (5% or 5 g yeast extract works even better)
125 ml Ustilago maydis salt solution (see below)
H2O to 1 L (pH 7.0)
15 g agar
5 g activated charcoal and stir
Autoclave at 121 °C, 15 psi for 15 min
After autoclaving add:
10 ml filter sterilized 50% glucose - Ustilago maydis salt solution
16 g KH2PO4
4 g Na2SO4
8 g KCl
2 g MgSO4.7H2O
1 g CaCl2.2H2O
8 ml trace element solution
H2O to 1 L
Storage at 4 °C - Trace element solution
30 mg H3BO3
70 mg MnCl2
200 mg ZnCl2
20 mg Na2MO4.2H2O
50 mg FeCl3
200 mg CuSO4
H2O to 500 ml
Storage at 4 °C
Acknowledgments
We are grateful to our funding sources including the US Department of Agriculture and National Science Foundation competitive grant programs. The methods described here for teliospore harvesting and germination and progeny isolation were adapted from standard protocols (Holliday, 1974).
References
- Alexopoulos, C. J. and Mims, C. W. et al. (1996). Chapter 21. Phyllum Basidiomycota. Order Ustilaginales. The Smut Fungi. In: Alexopoulos, C. J., Mims, C. W. and Blackwell, M. (eds). Introductory Mycology. Wiley, 639-657.
- Andrews, D. L., Egan, J. D., Mayorga, M. E. and Gold, S. E. (2000). The Ustilago maydis ubc4 and ubc5 genes encode members of a MAP kinase cascade required for filamentous growth. Mol Plant Microbe Interact 13(7): 781-786.
- Bölker, M., Genin, S., Lehmler, C. and Kahmann, R. (1995). Genetic regulation of mating and dimorphism in Ustilago maydis. Can J Bot 73(S1), 320-325.
- Holliday, R. (1974). Ustilago maydis. Chapter: Bacteria, Bacteriophages, and Fungi, In: King, R. C. (ed.) Handbook of genetics. Springer, 575-595.
- Nadal, M. and Gold, S. E. (2010). The autophagy genes ATG8 and ATG1 affect morphogenesis and pathogenicity in Ustilago maydis. Mol Plant Pathol 11(4): 463-478.
- Snetselaar, K. M., Carfioli, M. A. and Cordisco, K. M. (2001). Pollination can protect maize ovaries from infection by Ustilago maydis, the corn smut fungus. Can J Bot 79(12): 1390-1399.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Nadal, M., Takach, J. E., Andrews, D. L. and Gold, S. E. (2016). Mating and Progeny Isolation in the Corn Smut Fungus Ustilago maydis. Bio-protocol 6(8): e1793. DOI: 10.21769/BioProtoc.1793.
Category
Plant Science > Plant immunity > Host-microbe interactions
Microbiology > Microbial genetics > Gene mapping and cloning
Molecular Biology > DNA > DNA cloning
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









