- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Primary Cell Culture of Mouse Dorsal Root Ganglion Neurons
Published: Vol 6, Iss 7, Apr 5, 2016 DOI: 10.21769/BioProtoc.1785 Views: 33830
Reviewed by: Xuecai GeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3776 Views

Derivation and Culture of Enriched Phrenic-Like Motor Neurons From Human iPSCs
Louise Thiry [...] Stefano Stifani
Jul 5, 2025 2290 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2638 Views
Abstract
We here provide a detailed protocol for the isolation and culture of primary mouse sensory neurons. The cell bodies of sensory afferent pseudounipolar neurons are located in dorsal root ganglia (DRGs) along the vertebral column. Dissected mouse DRGs can be dissociated into single cells by enzymatic digestion to obtain primary cultures of mouse sensory neurons as performed in the studies reported by Khaminets et al. (2015).
Materials and Reagents
- T25 cell culture flask (25 cm2) (Greiner Bio-One GmbH, catalog number: 690160 )
- 24-well plate (Greiner Bio-One GmbH, catalog number: 662160 )
- Cover glasses (Marienfeld-Superior, catalog number: 0111550 )
- Glass Pasteur pipettes (Marienfeld-Superior, catalog number: 3233050 )
- Filtropur S 0.2 syringe filters (Sarstedt AG, catalog number: 83.1826.001 )
- Syringe Omnifix® (B. Braun Medical, catalog number: 4616103V )
- Dissection dish (petri dish) (94 x 16 mm) (Sigma-Aldrich, catalog number: Z617636 ) with silicone pad
- Greiner Petri dishes (35 x 10 mm) (Sigma-Aldrich, catalog number: P5112 )
- 15 ml Cellstar centrifugation tubes (Greiner Bio-One GmbH, catalog number: 188261 )
- Mice (2 to 6 months) (strain: C57BL/6)
- Poly-L-lysine hydrobromide (Sigma-Aldrich, catalog number: P2636 )
- HEPES (AppliChem GmbH, catalog number: A3268 )
- H3BO3 (Carl Roth GmbH + Co., catalog number: 6943.1 )
- Sodium tetraborate (Na2B4O7) (Sigma-Aldrich, catalog number: 221732 )
- Bovine Serum Albumin (PAA, catalog number: K41-001 )
- HBSS (Thermo Fisher Scientific, GibcoTM, catalog number: 14175-053 )
- Neurobasal®-A medium (Thermo Fisher Scientific, GibcoTM, catalog number: 10888022 )
- B-27® Supplement (50x) (Thermo Fisher Scientific, GibcoTM, catalog number: 17504-044 )
- L-glutamine (200 mM) (Thermo Fisher Scientific, GibcoTM, catalog number: 25030 )
- Penicillin/streptomycin (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140 )
- Minimum Essential Medium (MEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 31095-029 )
- D(+)-Glucose (C6H12O6) (Merck Millipore Corporation, catalog number: 108337 )
- Collagenase-II (Worthington Biochemical Corporation, catalog number: LS004176 )
- Horse serum (Thermo Fisher Scientific, GibcoTM, catalog number: 26050-88 )
- Trypsin from bovine pancreas (Sigma-Aldrich, catalog number: T1426 )
- β-nerve growth factor (β-NGF) (Preprotec, catalog number: 450-01 )
- Borate buffer (see Recipes)
- Poly-L-lysine hydrobromide (PLL) stock solution (see Recipes)
- Poly-L-lysine hydrobromide (PLL) working solution (see Recipes)
- Dissociation solution (see Recipes)
- 30% Glucose stock solution (see Recipes)
- 0.5% BSA-blocking solution (see Recipes)
- DRG neuronal culture medium (see Recipes)
- DRG preparation medium (see Recipes)
- Collagenase-II solution (see Recipes)
- 2% trypsin stock solution (see Recipes)
Equipment
- CO2 incubator (37 °C, 5% CO2 concentration and 95% relative humidity) (Labotect, model: C-200 )
- Laminar flow work bench (Heraeus Holding)
- Stereo microscope (Carl Zeiss, model: Stemi 2000C )
- Cold light source (equipped with a flexible dual branch light guide) (Carl Zeiss, model: CL1500ECO )
- Scissors
- Forceps
- Bunsen burner Fireboy plus (Integra Biosciences)
- Glass Pasteur pipettes (Marienfeld-Superior, catalog number: 3233050 )
- Dropper bulb for Pasteur pipettes (Thermo Fisher Scientific, catalog number: 03-448-25 )
- Centrifuge 5810R (Eppendorf AG)
- Pipetman P20/P200/P1000 (Gilson)
Procedure
- Day 1: Dissociation solution is prepared and precooled to 4 °C.
- A T25 cell culture flask is coated with Poly-L-lysine hydrobromide (PLL). For this purpose 1 ml of PLL solution (1 mg/ml in borate buffer) is added to the cell culture flask and incubated overnight at 37 °C. Alternatively, eight cover glasses are placed into the wells of a sterile 24-well plate and incubated with PLL overnight at 37 °C.
- Day 2: PLL solution is aspirated and the cell culture flask is rinsed three times with sterile water. The flask is left open for drying in a laminar flow hood for 15 min.
- Then the flask is pre-incubated with 5 ml of DRG neuronal culture medium and placed into the incubator.
- Sterile glass pipettes are blocked by pipetting up and down 0.5% BSA-blocking solution three times. Blocked glass Pasteur pipettes are stored in the laminar flow work bench till usage at the same day.
- The scissors and forceps are disinfected for 30 min in 70% ethanol followed by air drying.
- The mouse is killed via cervical dislocation and the spinal cord is dissected rapidly with a pair of standard scissors. To prepare the spinal cord, the skin is incised at the ventral medial line with the standard scissors. Organs are removed from the thoracic and abdominal cavity. Using the standard scissors, two long cuts are made closely left and right to the spinal column. The tail and skull are removed. At last, remaining paravertebral muscles are largely removed to expose the spinal column.
- Using a stereo microscope, DRGs from all spinal levels are carefully removed and collected in 10 ml of HBSS in a 10-cm-dissecting dish placed on ice. In detail, the spine is cut first into three approximately equal pieces with the student spring scissors to improve subsequent handling. Then, one blade of the student spring scissors is inserted from the ventral side into the spinal canal. The other blade of the scissors is placed outside the spinal column on its ventral side. Two incisions are made along the spine, one left and one right of the midline on the ventral side as illustrated in Figure 1A-C and the scheme of Figure 2. The excised part of the spine is lifted and the spinal marrow is removed from the column (see Figure 1D-F). DRGs are clusters of somata of sensory neurons and are located closely along the dorsal root of the cord. DRGs are lying in the intervertebral foramina and have a long distal and proximal process (pseudounipolar neurons). DRGs are characterized by a round shape and hyaline appearance that differs from the white color of nerve fiber bundles. The student spring scissors, Dumont #7b and #4 forceps are used to carefully isolate the exposed ganglia for each of the three pieces of the spinal cord.
- Isolated DRGs are collected in a 35-mm-petri dish filled with 5 ml of DRG preparation medium. The dish is kept on ice. Approximately 20 to 40 ganglia are obtained according to the expertise of the experimenter.
- The thin layer of connective tissue of the epineurium surrounding the ganglion is removed with the help of a pair of spring scissors and the Dumont #5 Mirror Finish Forceps. Then the DRGs are transferred to a second 35-mm dish filled with DRG preparation medium on ice.
- Collagenase-II solution is prepared and preheated for 10 min to 37 °C.
- Isolated DRGs are transferred into a 15-ml-centrifugation tube with the help of the glass Pasteur pipette under a laminar flow workbench.
- After DRGs are settled on the tube bottom, the supernatant is removed and discarded.
- DRGs are rinsed with 5 ml of precooled and sterile dissociation solution.
- Again, the supernatant is removed. As described in steps 13 and 14, DRGs are washed three times in total, each with 5 ml of dissociation solution.
- After the last washing step, 2 ml of dissociation solution are left and 1 ml of Collagenase-II solution is added.
- DRGs are incubated for 1 h at 37 °C while gently shaking the Falcons every 10 min.
- An aliquot of the 2%-trypsin stock solution is defrosted and preheated for 20 min to 37 °C.
- 150 µl of activated trypsin is added.
- The DRGs are incubated for further 9 min at 37 °C while gently shaking the falcon every 3 min.
- The supernatant is removed carefully and washed with 5 ml of dissociation solution as described in steps 13 and 14.
- After the last washing step, 1.5 ml of dissociation solution are left.
- To obtain single cells, DRGs are dissociated with the glass Pasteur pipettes that had been prepared in step 5. Two glass Pasteur pipettes are fire-polished to obtain smaller openings. Using the glass pipette with an original opening diameter of 1.1-1.3 mm DRGs are pipetted approximately 10 times up and down till the solution appeared homogenous. Then the (fire-polished) Pasteur pipette with the smaller diameter is used and the solution triturated 8 times. Finally, the very small diameter (fire-polished) Pasteur pipette is used 3 times.
- The single cell suspension is centrifuged for 5 min at 160 x g. The deceleration of the centrifuge is reduced to ‘3’ (out of 10 braking ramps) in order to avoid that the small and fragile cell pellet gets dispersed while the centrifuge is stopped.
- The supernatant is removed from the cell pellet.
- DRG neuronal culture medium supplemented with 10% horse serum is added to the cell pellet; cells are resuspended and centrifuged for 5 min at 160 x g. The deceleration of the centrifuge is again set to ‘3’.
- The supernatant is carefully removed and cells are resuspended in DRG neuronal culture medium with a 1,000 µl pipette.
- Cells obtained from one mouse (approximately 50,000 to 100,000 cells) are plated in one T25 cell culture flask or alternatively on eight cover glasses (10 mm diameter) and placed at 37 °C under a 5% CO2 atmosphere.
- 30 min after plating, medium is replaced by fresh DRG cell culture medium supplemented with β-NGF (50 ng/ml). A volume of 5 ml is added to a T25 flask or 0.5 ml for each well of a 24-well plate.
- Primary cultures are maintained for up to three weeks. One third of the volume of DRG neuronal culture medium freshly supplemented with β-NGF (50 ng/ ml) is replaced every 3 to 5 days. Figure 3 shows bright field microscopy images of cultured DRG neurons at different time points after plating demonstrating the progressive growth of DRG neuronal processes in culture. Staining for the neuron-specific class III beta-tubulin (see Figure 4) further illustrates the characteristic morphology of cultured DRG neurons stimulated by β-NGF supplemented to the culture medium.
Representative data

Figure 1. Illustration of DRG dissection. A. The ventral side of the spinal cord is shown. B-C. Two incisions are made along the ventral side of the spinal cord, one left (B) and one right (C). D-E. The excised part of the spine is lifted. F. The spinal marrow is removed from the column. G. Ganglia are exposed and (H) carefully isolated from the surrounding tissue. I. The arrow points to an isolated dorsal root ganglion with its characteristic round shape, hyalin appearance and part of its proximal process. 
Figure 2. Schematic illustration of spinal cord preparation for DRG isolation. For opening of the spinal canal two incisions are done (red dotted lines). The location of sensory ganglia at the dorsal side is shown.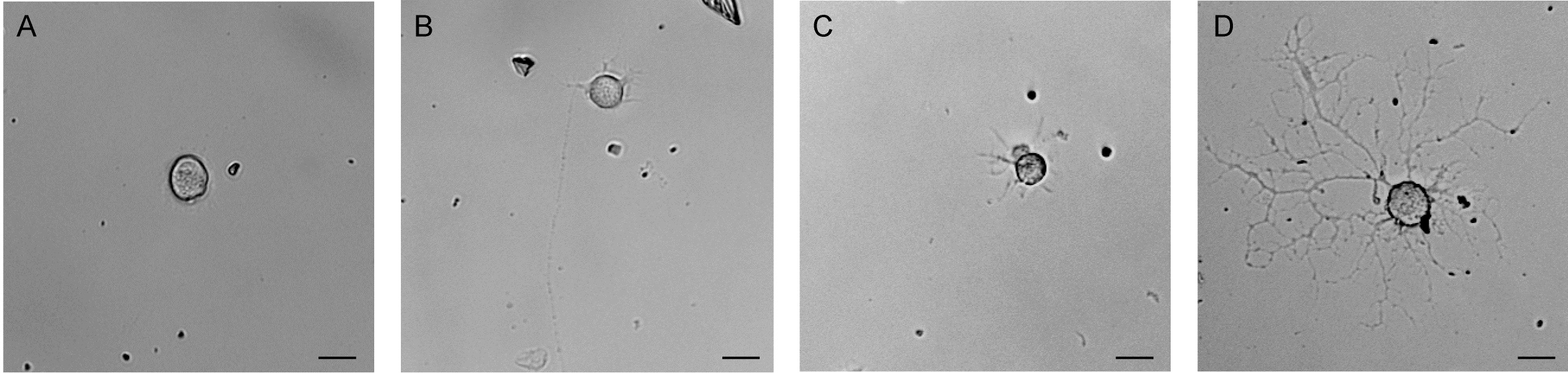
Figure 3. Dissociated primary DRG neurons in culture. Brightfield images of primary mouse dorsal root ganglion neurons (A) three hours after plating, (B) at day 1 in vitro, (C) at day 2 in vitro and (D) at day 5 in vitro. Scale bars: 10 µm
Figure 4. Confocal images of fluorescently labeled primary DRG neurons. A. Primary mouse dorsal root ganglia neurons after 4 days in culture. Fixed cells are stained with neuron-specific class III beta-tubulin (Clone TUJ1, Stemcell technologies, 1:500). B. Nuclei are stained with Hoechst 33258. C. Merged image. Scale bars: 100 µm
Recipes
- Borate buffer
1.24 g H3BO3
1.90 g Na2B4O7
Add ddH2O to 400 ml
Stored at 4 °C for up to 1 year - Poly-L-lysine hydrobromide (PLL) stock solution
Dissolve 100 mg of PLL in 10 ml of ddH2O
Aliquot and stored at -20 °C for up to 6 months - Poly-L-lysine hydrobromide (PLL) working solution
Mix 1 ml of PLL stock solution (10 mg/ml) with 9 ml of borate buffer
Filter sterilize (0.20 µm-filter) and stored at 4 °C for up to 4 weeks - Dissociation solution
Mix 500 ml of HBSS with 3.5 ml of HEPES (1 M, pH 7.25)
Add 5 ml penicillin/streptomycin (100x)
Solution was freshly prepared for each experiment - Glucose stock solution (30%)
Dissolve 30 g of C6H12O6 in 50 ml of ddH2O
Add ddH2O to 100 ml
Filter sterilize (0.20 µm-filter) and stored at 4 °C for up to 6 months - 0.5% BSA-blocking solution
0.5 g of BSA
Add HBSS to 100 ml
Filter sterilize (0.20 µm-filter) and stored at 4 °C for up to 1 year - DRG neuronal culture medium
100 µl L-glutamine (200 mM)
400 µl B-27® Supplement (50x)
200 µl penicillin/streptomycin (100x)
Add Neurobasal®-A medium to 20 ml
Stored at 4 °C for up to 2 weeks - DRG preparation medium
140 µl HEPES (1 M, pH 7.25)
200 µl glucose stock solution (30%)
100 µl penicillin/streptomycin (100x)
Add Minimal Essential Medium (MEM) to 10 ml
Medium was freshly prepared for each experiment - Collagenase-II solution
Dissolve 10 mg of collagenase-II in 1 ml of dissociation solution
Solution was freshly prepared for each experiment - 2% trypsin stock solution
Dissolve 100 mg of trypsin in 5 ml of HBSS
Aliquot and stored at 4 °C for up to 6 months
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft to I. K. (KU 1587/4-1), C. A. H. (HU 800/6-1, RTG1715), T. H. (RTG 1715). Animal care and experimental procedures were performed in accordance with the guidelines established by the animal welfare committee of the University of Jena.
References
- Khaminets, A., Heinrich, T., Mari, M., Grumati, P., Huebner, A. K., Akutsu, M., Liebmann, L., Stolz, A., Nietzsche, S., Koch, N., Mauthe, M., Katona, I., Qualmann, B., Weis, J., Reggiori, F., Kurth, I., Hubner, C. A. and Dikic, I. (2015). Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522(7556): 354-358.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Heinrich, T., Hübner, C. A. and Kurth, I. (2016). Isolation and Primary Cell Culture of Mouse Dorsal Root Ganglion Neurons. Bio-protocol 6(7): e1785. DOI: 10.21769/BioProtoc.1785.
Category
Neuroscience > Sensory and motor systems > Cell isolation and culture
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









