- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Strategies for Performing Dynamic Gene Perturbation Experiments in Flowers
Published: Vol 6, Iss 7, Apr 5, 2016 DOI: 10.21769/BioProtoc.1774 Views: 9956
Reviewed by: Samik BhattacharyaXinyan ZhangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification of Insertion Site by RESDA-PCR in Chlamydomonas Mutants Generated by AphVIII Random Insertional Mutagenesis
Fantao Kong and Yonghua Li-Beisson
Feb 5, 2018 8592 Views

High Resolution Melting Temperature Analysis to Identify CRISPR/Cas9 Mutants from Arabidopsis
Cynthia Denbow [...] Sakiko Okumoto
Jul 20, 2018 8939 Views

Dual sgRNA-based Targeted Deletion of Large Genomic Regions and Isolation of Heritable Cas9-free Mutants in Arabidopsis
Yu Jin and Sebastian Marquardt
Oct 20, 2020 6772 Views
Abstract
Dissecting the gene regulatory networks (GRNs) underlying developmental processes is a central goal in biology. The characterization of the GRNs underlying flower development has received considerable attention, however, novel approaches are required to reveal temporal and spatial aspects of these GRNs. Here, we provide an overview of the options available to perform dynamic gene perturbations to identify downstream response genes at specific stages of development in the flowers of Arabidopsis thaliana.
Keywords: Arabidopsis thaliana[Introduction] Gene activity perturbation followed by expression analysis of downstream targets represents a powerful method to understand gene function and dissect regulatory hierarchies. The transcriptomes of “static” mutant lines (e.g., caused by point mutations, transfer-DNA insertions, or constitutive expression) are often compared with those of wild type counterparts, which can confound the biological interpretation of the expression data. For example, a null mutant (noted ag-1) of the transcription factor AGAMOUS (AG) lacks stamens and carpels, which are replaced by sepals and petals (Figure 1A and 1B) (Bowman et al., 1989). A comparison of the transcriptomes of ag-1 flowers with wild-type flowers may lead to the conclusion that AG regulates the expression of genes controlling the specification of mature tissues in the anther and gynoecium. This interpretation is inappropriate, as AG is required for the specification of these organ-types (Bowman et al., 1989; Yanofsky et al., 1990). Therefore, the cause of the expression differences observed may be a result of tissue biases (Wellmer et al., 2004). Dynamic perturbations circumvent these and other issues: i) the tissues harvested will be morphologically identical, as tissue from “mock-treated” and inducer-treated plants would normally be harvested within a 24 h time-frame, ii) the response of downstream targets can be measured in the range of minutes and hours, which can be useful to infer direct and indirect interactions, iii) the development-dependent functions of the regulators of interest can be dissected (O'Maoileidigh et al., 2015). Flowers of the model plant Arabidopsis thaliana are minute, particularly at early stages of development. Furthermore, they are initiated in a sequential manner such that no two flowers are at the exact same stage of development on any given inflorescence (Smyth et al., 1990). Until recently, these traits have inhibited investigations into the molecular mechanisms underlying the earliest stages of flower development. There are many strategies available to isolate tissue from young flower buds including laser capture microdissection and fluorescent activated cell sorting (Birnbaum et al., 2003; Mantegazza et al., 2014; Wuest and Grossniklaus, 2014). We prefer to use a floral induction system (FIS) to isolate flowers at specific stages of development, as it is a user friendly, low-cost approach (O'Maoileidigh et al., 2015; Wellmer et al., 2006; O'Maoileidigh and Wellmer, 2014). This system is based on the reintroduction of APETALA1 (AP1) activity into the apetala1-1 cauliflower-1 (ap1-1 cal-1) background via either a rat glucocorticoid (Wellmer et al., 2006; O'Maoileidigh and Wellmer, 2014; O’Maoileidigh et al., 2013) or a mouse androgen (O'Maoileidigh et al., 2015) receptor ligand binding domain (noted GR and AR, respectively) fusion with AP1. Once the FIS ap1-1 cal-1 inflorescences are treated with dexamethasone or dihydrogentestosterone (DHT, 5α-androstan-17β-ol-3-one), respectively, AP1 translocates to the nucleus to regulate transcription, which leads to the formation of many flowers on a single inflorescence that are at similar stages of development (Wellmer et al., 2006; O'Maoileidigh and Wellmer, 2014). We have shown that stage-specific flowers can be reliably harvested from early to late stages of development (Wellmer et al., 2006; Ryan et al., 2015). We have also demonstrated that these FISs can be combined with two-component inducible systems that facilitate dynamic perturbations (O'Maoileidigh et al., 2015; O’Maoileidigh et al., 2013; Wuest et al., 2012). We have generated FISs that are responsive to dexamethasone and DHT, which we have combined with the ethanol-responsive AlcApro/AlcR and the OPpro/GR-LhG4 dexamethasone-responsive two-component systems, respectively, to express artificial microRNAs (amiRNAs) that perturb gene activity. We have successfully used these lines to assess the stage-specific functions of genes of interest (O'Maoileidigh et al., 2015; O’Maoileidigh et al., 2013; Wuest et al., 2012). Notably, we also observed that these two-component inducible systems can influence the expression of the A. thaliana genome independently of the intended amiRNA-mediated perturbation (O'Maoileidigh et al., 2015). Therefore, we developed and described several control measures that must be taken in order to account for these experimental artifacts (O'Maoileidigh et al., 2015). In the following protocol, we provide technical advice for implementing dynamic perturbation strategies during flower development and the establishment of the experimental design. As a guide, we briefly discuss the perturbation strategies implemented to understand the functions of the homeotic gene AG, including our published and unpublished results, as well as data from the literature.
Materials and Reagents
- 20 cm x 32 cm x 50 cm trays with plastic lids [Romberg & Sohn, catalog number: 51221K (trays) and 74051K (lids)]
- 50 ml centrifuge tubes (Sigma-Aldrich, catalog number: CLS430829-500E )
- Disposable pasteur pipettes (Thermo Fisher Scientific, catalog number: 12837625 )
- Mouse AR coding sequence [obtained from the vector pBJ36-mAR (O'Maoileidigh et al., 2015)]
Note: Only the ligand-binding domain was fused to AP1. - Rat GR coding sequence [obtained from the vector pBJ36-GR (O’Maoileidigh et al., 2013)]
Note: Only the ligand-binding domain was fused to AP1. - SRDX domain fusions [produced using the vector p35SSRDXG (Mitsuda et al., 2011)]
Note: The SRDX amino acid sequence is LDLELRLGFA (Ikeda and Ohme-Takagi, 2009). - The WUSB coding sequence [obtained from the vector p35SWUSB (Ikeda et al., 2009)]
Note: The WUSB amino acid sequence is HRRTLPLFPMHGED (Ikeda et al., 2009). - VP16 domain fusions [produced using the vector p35SVP16 (Mitsuda et al., 2011)]
Note: Genbank accession KM486811 for VP16 activation domain sequence in a cloning vector. - AlcA sequence [obtained from AlcApro-pBJ36 (Leibfried et al., 2005)]
- AlcR coding sequence [obtained from 35Spro:AlcR-pML-BART (Leibfried et al., 2005)]
- GR-LhG4 coding sequence [obtained from 35Spro:GR-LhG4-pML-BART (Wuest et al., 2012)]
- OPpro sequence [obtained from 6xOPpro-pBJ36 (Wuest et al., 2012)]
- Floral induction system (FIS) [Various versions of the FISs are available from Dr. Frank Wellmer (O'Maoileidigh et al., 2015; Wellmer et al., 2006; O'Maoileidigh and Wellmer, 2014)]
- CONTROL-amiRNA sequence [obtained from pBJ36-AlcApro:CTRL-amiRNA (O'Maoileidigh et al., 2015)]
- GR antibody [obtained from Santa Cruz ( sc-1002 ) (Kaufmann et al., 2010)]
- Dexamethasone (Sigma-Aldrich, catalog number: D4902 )
- α-Androstan-17β-ol-3-one (DHT) (Sigma-Aldrich, catalog number: A8380 )
- General reagents for molecular cloning and expression analysis
- Liquid nitrogen
- 10 mM dexamethasone stock solution (see Recipes)
- 100 mM DHT stock solutionpowder (see Recipes)
- GR activation solution (see Recipes)
- AR activation solution (see Recipes)
Equipment
- Sharp Forceps (Sigma-Aldrich, catalog number: T5790 )
- Dissection microscope
- General equipment for molecular cloning and expression analysis
Procedure
- Gain-of-function-mediated dynamic perturbation
The use of inducible gain-of-function strategies to understand regulatory hierarchies can be extremely informative. For example, Gomez-Mena et al. (2005) overexpressed an AG-GR fusion under the control of the Cauliflower Mosaic Virus (CMV) 35S promoter (Benfey and Chua, 1990), to identify targets of AG (Gomez-Mena et al., 2005). Many of the genes they identified as differentially expressed in response to AG activation were shown to be direct targets (O’Maoileidigh et al., 2013; Gomez-Mena et al., 2005). Ito et al. (2004) also used a 35Spro:AG-GR line to perform perturbation experiments in the presence of the protein biosynthesis inhibitor cycloheximide, which was used to infer the direct regulation of the SPOROCYTELESS gene by AG. In addition, Ito et al. (2007) introduced the 35Spro:AG-GR transgene into the ag-1 background and determined the temporal requirements for AG activity, particularly during stamen development, using an AG dosage-dependent rescue assay (Ito et al., 2004). Although these gain-of-function-mediated perturbation strategies are extremely useful, they can also produce artifactual results, as the activity of the protein of interest may behave differently due to its altered spatio-temporal pattern of expression (Smith et al., 2011).
To perform a gain-of-function-mediated dynamic perturbation experiment, the protein of interest can simply be expressed from a promoter of interest in combination with the glucocorticoid (GR) or androgen (AR) receptor fusion technique (O'Maoileidigh et al., 2015; Yamaguchi et al., 2015; Sun et al., 2009). Alternatively, the chemically inducible two-component promoter systems OPpro/GR-LhG4 or AlcApro/AlcR can be employed (Roslan et al., 2001; Deveaux et al., 2003; Craft et al., 2005). Use of these promoter systems would not require modification of the protein of interest, which simplifies the design process relative to the GR/AR fusion techniques and eliminates the possibility that the GR/AR domain will interfere with protein function.- Fuse the coding region of your gene of interest to the coding region of the GR/AR ligand binding domain (Notes 1-2). Be sure to preserve the open reading frames of the gene of interest and the GR/AR domain. Place the gene fusion downstream of a promoter of choice (Note 3) in a binary vector. Alternatively, utilize a two-component inducible system (see above and Note 4).
- Transform wild-type or mutant plants (Note 5) with the inducible transgene by floral dip and identify transformants using the appropriate selection technique (Clough and Bent, 1998).
- Discard first generation (T1) plants displaying phenotypes in the absence of the inducing agent.
- Treat the inflorescences of the remaining first generation plants with the appropriate inducer (Note 6).
- Phenotype the flowers at least every second day after treatment. A gradient of phenotypes would be expected, depending on the stage of a given flower when it was treated (Note 7).
- Alternatively, the plants can also be characterized on a molecular level to select candidates for further analysis (Notes 8-9). However, the phenotype produced after activating the transgene should be carefully examined.
- Select several independent lines and, if using the GR/AR fusion technique, perform Western blotting analysis, using an anti-GR or anti-AR antibody, in the second generation to determine the level of expression of the fusion protein.
- Select lines that produce full-length proteins, desirable phenotypes and/or desirable mRNA levels. Isolate lines that are homozygous for the transgene.
- At this point, an inducible perturbation experiment can be initiated. However, it may be difficult to dissect the stage-specific functions of the factor of interest on a molecular level. Therefore, we recommend crossing the transgenic lines with the appropriate floral induction system.
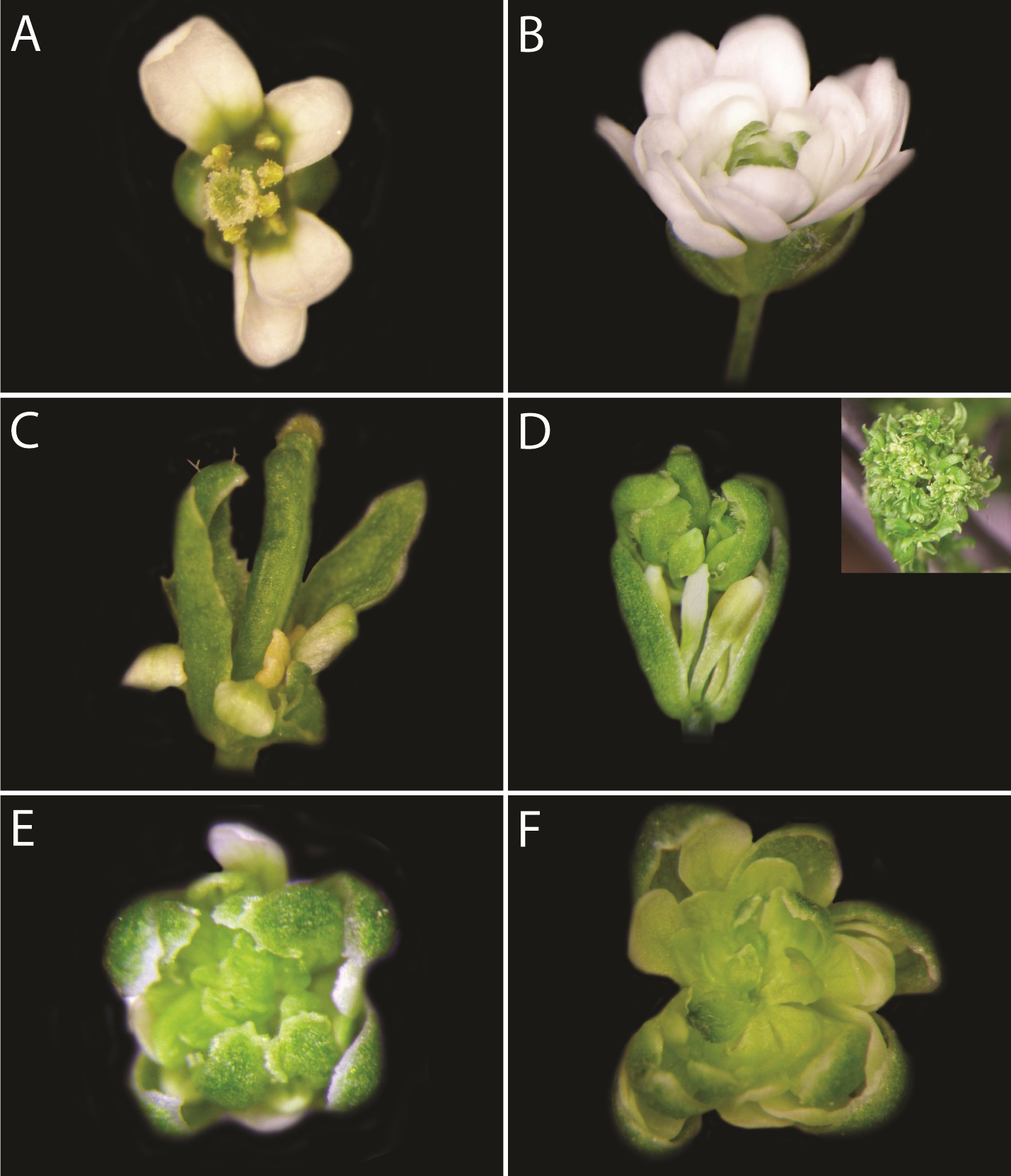
Figure 1. Perturbation of AG activity. A. A wild-type Landsberg erecta flower; B. An ag-1 flower; C. A flower from a plant containing a 35Spro:AG-SRDX transgene (unpublished); D. A flower from a plant containing a 35Spro:AG-WUSB transgene (unpublished). Inset: An inflorescence from an independent line containing the 35Spro:AG-WUSB transgene (unpublished); E. A flower from a plant containing a 35Spro:RNAi-AG transgene (unpublished); F. A flower from a plant containing a 35Spro:AG-4-amiRNA transgene (O’Maoileidigh et al., 2013).
- Fuse the coding region of your gene of interest to the coding region of the GR/AR ligand binding domain (Notes 1-2). Be sure to preserve the open reading frames of the gene of interest and the GR/AR domain. Place the gene fusion downstream of a promoter of choice (Note 3) in a binary vector. Alternatively, utilize a two-component inducible system (see above and Note 4).
- Transcriptional effector domain-mediated dynamic perturbation
The function of the protein of interest can be modified by fusing it to a transcriptional repressor domain. For example, overexpression of a fusion between AG and an ERF-associated amphiphilic repression (EAR) motif from the protein SUPERMAN (SRDX) was shown to phenocopy a strong ag mutant phenotype (Mitsuda et al., 2006). We independently generated a 35Spro:AG-SRDX fusion that was designed to produce an identical protein to the published version in Mitsuda et al. (2006) (Figure 1C), however, we did not observe any strong ag-like phenotypes. We also overexpressed a fusion of AG with the WUSB repression domain from the transcription factor WUSCHEL in plants (Ikeda et al., 2009) and, in this case, did observe ag-like phenotypes (Figure 1D). However, we also observed plants with leaf-like floral organs (Figure 1D, inset). The reasons behind these discrepancies are unclear to us, however, they highlight the usefulness of generating control lines that constitutively express the chimeric gene prior to generating an inducible version.
The gene of interest can also be expressed as fusion with a transcriptional activation domain. To our knowledge, such domains have not been fused with AG, however, they have been successfully used to understand the functions of other important floral regulators (e.g., Parcy et al., 1998). As with the gain-of-function experiments, the overexpression of the chimeric protein may alter its behavior.
To perform this type of perturbation, the protein of interest can be fused to a transcriptional activation [e.g., VP16 (Triezenberg et al., 1988; Cousens et al., 1989)] or repression domain [e.g., SRDX (Hiratsu et al., 2002)]. This should result in the activation or repression of all genes that the protein associates with, respectively (Note 10). The choice of an activation or repression domain would be weighted based on the known activities of the protein of interest and on the presence of endogenous transcriptional effector domains in the protein of interest.- Fuse the protein of interest to the transcriptional effector domain of choice so that the reading frame of the gene and domain remain intact. The chosen domain should be positioned so that it is unlikely to interfere with the activity of the protein of interest (Notes 1-2). To generate a non-inducible version of the fusion protein, place this fusion gene downstream of a chosen promoter (Note 11) in a binary vector.
- Transform wild-type plants with the fusion gene by floral dip (Clough and Bent, 1998) and identify transformants using the appropriate selection technique (Miki and McHugh, 2004).
- Characterize the phenotypes of the resulting transgenic plants. If they match expectations, proceed to step B4 (Note 12).
- To make an inducible version, introduce the fusion gene into the dexamethasone or ethanol-responsive two-component promoter systems (Note 4).
- Transform wild-type plants with inducible transgene by floral dip (Clough and Bent, 1998) and identify transformants using the appropriate selection technique (Miki and McHugh, 2004).
- Discard T1 plants displaying phenotypes in the absence of the inducing agent.
- Treat the inflorescences of the remaining T1 plants with the appropriate inducer (Note 6).
- Phenotype the flowers periodically after treatment. A gradient of phenotypes would be expected, depending on the stage of a given flower when it was treated (Note 7).
- Alternatively, the plants can also be characterized on a molecular level to select candidates for further analysis (Notes 8-9). However, the phenotype produced after activating the transgene should be carefully examined.
- Select plants that produce desirable phenotypes and/or desirable mRNA levels and isolate lines that are homozygous for the transgene.
- At this point, an inducible perturbation experiment can be initiated. However, it may be difficult to dissect the stage-specific functions of the factor of interest on a molecular level. Therefore, we recommend crossing the transgenic lines with the appropriate floral induction system.
- Fuse the protein of interest to the transcriptional effector domain of choice so that the reading frame of the gene and domain remain intact. The chosen domain should be positioned so that it is unlikely to interfere with the activity of the protein of interest (Notes 1-2). To generate a non-inducible version of the fusion protein, place this fusion gene downstream of a chosen promoter (Note 11) in a binary vector.
- Loss-of-function-mediated dynamic perturbation
The use of loss-of-function-mediated perturbation strategies offers several advantages over the perturbation strategies described above. In this case, the activity of the factor of interest would be removed via small RNAs. Therefore, the caveats associated with overexpression of the factor of interest, or a modified version, would not apply. The options available to perform this type of perturbation include the production of short interfering RNAs from a double-stranded RNA (dsRNA) precursor (termed RNA interference, RNAi) or artificial microRNAs (amiRNAs) (Ossowski et al., 2008; Schwab et al., 2006). The RNAi strategy relies on expressing a dsRNA that is homologous to a large portion of the factor of interest. This results in the production of many short RNA molecules, whereas amiRNAs rely on the production of a single type of small RNA to target a specific transcript (Schwab et al., 2006). Notably, RNAi-mediated perturbation is thought to lead to more off-targets than amiRNA-mediated perturbation (Ossowski et al., 2008; Schwab et al., 2006).
We previously screened seven independent amiRNA constructs before identifying an amiRNA that efficiently targets the AG mRNA, which, when overexpressed, can recapitulate a strong ag null phenotype (Figure 1F) (O’Maoileidigh et al., 2013). We also successfully perturbed AG activity with an RNAi construct, however, the phenotype was somewhat weaker than the phenotype observed for the functional amiRNA (Figure 1E). Furthermore, the proportion of first generation plants that displayed a phenotype in the RNAi lines (~8%) was much lower than the proportion of lines that displayed a phenotype in the amiRNA lines (100%). Given this, and the potential off-target effects associated with RNAi-mediated perturbation, our recommendation would be to utilize amiRNAs to perturb gene activity.
To perform a loss-of-function-mediated dynamic perturbation experiment, the chemically inducible two-component promoter systems described above can be used. To demonstrate functionality, we recommend making stable transformants that constitutively express the RNAi/amiRNA of interest prior to making an inducible version (Note 13).- Design an RNAi (Note 14) or amiRNA (Note 15) construct and place it under the control of a promoter of choice in a binary vector.
Notes:- The materials and the cloning procedure to generate RNAi constructs are described in Eamens and Waterhouse (2011).
- The cloning procedure to generate amiRNAs is described by Schwab et al. (2006) while the appropriate vectors can be obtained through wmd3.weigelworld.org.
- The materials and the cloning procedure to generate RNAi constructs are described in Eamens and Waterhouse (2011).
- Transform wild-type plants with the construct by floral dip (Clough and Bent, 1998) and identify transformants using the appropriate selection technique (Miki and McHugh, 2004).
- If the phenotypes of the transgenic plants match expectations, proceed to step C4 (Note 12). Alternatively, if the phenotype of a corresponding null mutant is unknown, the mRNA levels of the gene of interest can be determined.
- To allow an inducible knockdown of gene activities, introduce the RNAi/amiRNA sequence into the dexamethasone or ethanol responsive two-component promoter systems (Note 4).
- Transform wild-type plants with inducible transgene by floral dip (Clough and Bent, 1998) and identify transformants using the appropriate selection technique (Miki and McHugh, 2004).
- Discard T1 plants displaying phenotypes in the absence of the inducing agent.
- Treat the inflorescences of the remaining first generation plants with the appropriate inducer (Note 6).
- Phenotype the flowers periodically after treatment. A gradient of phenotypes would be expected, depending on the stage of a given flower when it was treated (Note 16).
- Alternatively, the plants can be characterized on a molecular level to select candidates for further analysis (Notes 8-9).
- Select plants that produce desirable phenotypes and/or desirable mRNA levels (Note 17) and isolate lines that are homozygous for the transgene.
- At this point, an inducible perturbation experiment can be initiated. However, it may be difficult to dissect the stage-specific functions of the factor of interest on a molecular level. Therefore, we recommend crossing the transgenic lines with the appropriate floral induction system.
- Design an RNAi (Note 14) or amiRNA (Note 15) construct and place it under the control of a promoter of choice in a binary vector.
- Inducible perturbation followed by gene expression analysis and phenotypic analysis
Gene expression profiling using dynamic perturbations with whole inflorescences
It is possible to perform an inducible gene perturbation experiment using whole inflorescences, however, no temporal information will be available from these data. In fact, the RNA populations isolated are likely to be dominated by RNA from older flowers (Wellmer et al., 2004).- Grow plants so that they bolt in a relatively synchronous manner (Note 18).
- Divide plants into two populations: those that will be treated with the inducer and those that will be treated with a mock solution. The same strategy would be applied to any control plants being used.
- Treat the inflorescence to activate expression of the dynamic perturbation transgene (Note 6).
- Harvest approximately 20 inflorescences per sample from a few hours to days after initiating the dynamic perturbation using liquid nitrogen to keep the samples frozen. Extract RNA and perform qRT-PCRs to measure the responsiveness of i) the effector molecule (e.g., amiRNA precursor, protein fusion) and ii) a gene (or several genes) that will be affected by the induction. These data will allow the assessment of the kinetics of the knockdown, which will inform the subsequent experimental design (Note 19).
- Select the time at which the tissue will be harvested after the perturbation construct is activated.

Figure 2. Response of AP1pro:AP1-GR ap1 cal inflorescence to dexamethasone treatment. A. An untreated inflorescence-like meristem. B-C. An inflorescence meristem 5 days (B) and 8 days (C) after treatment with a solution containing dexamethasone. - Repeat steps 1-3 in Section D, above.
- Harvest approximately 20 inflorescences at chosen time-points using liquid nitrogen to keep the samples frozen.
- Process tissue for molecular analysis.
Gene expression profiling in a stage-specific manner using dynamic perturbations
Combining dynamic perturbation strategies with the FIS, which facilitates the collection of flowers at similar stages of development, affords the user with a low-cost, easy to use approach to identify stage-specific functions of their gene of interest (Figure 2A-C). The inducible transgene of choice can be crossed with the appropriate FIS or can be transformed directly into the FIS (Notes 20-21).- Isolate the inducible transgene in a suitable FIS background (Note 20).
- Grow plants so that they bolt in a relatively synchronous manner (Note 18).
- Induce synchronous flowering by locally treating the ap1-1 cal-1 inflorescences (Note 22) (O'Maoileidigh et al., 2015).
- Divide plants into two populations: those that will be treated with the inducer and those that will be treated with a mock solution. The same strategy would be applied to any control plants being used.
- Treat the inflorescence to activate expression of the dynamic perturbation transgene (Note 6).
- Harvest whole flowers (Note 23) from approximately 20 plants per sample from hours to days after initiating the dynamic perturbation using liquid nitrogen to keep the samples frozen. Extract RNA and perform qRT-PCRs to measure the responsiveness of i) the effector molecule (e.g., amiRNA precursor, protein fusion) and ii) candidate gene(s) that will be affected by the perturbation to the inducer. These data will allow the assessment of the kinetics of the knockdown, which will inform the subsequent experimental design (Note 19).
- To perform the perturbation experiment, select the time-points of flower development you wish to investigate using Table 1.
- Select the time at which the tissue will be harvested after the perturbation construct is activated.
- Repeat steps 1-4 in Section D, above.
- Harvest whole flowers from approximately 20 plants at the chosen time-points from inducer-treated and mock-treated inflorescences using liquid nitrogen to keep the samples frozen.
- Process tissue for molecular analysis.
Table 1. Correlation of days after activation of synchronous flowering in the FIS with stages of flower development. After day 7-8, the flowers developing from the inflorescence are no longer synchronized. At this point, however, it is possible to distinguish them morphologically. The table indicates approximations of the developmental stages that can be harvested on the indicated day after initiation of synchronous flowering using the FIS. These approximations are based on the flower stages described in Smyth et al. (1990) and growth of the plants in continuous light.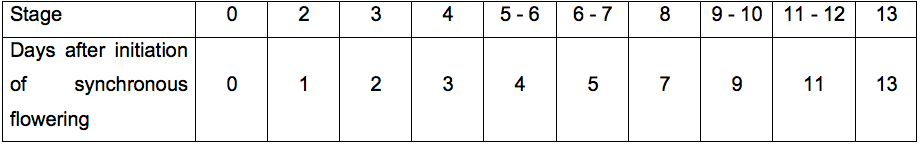
- Grow plants so that they bolt in a relatively synchronous manner (Note 18).
- Activate the inducible promoter system with the appropriate inducer (Note 6).
- Dissect flowers at anthesis in the following days and phenotype them as desired. Continue this process until the flowers have produced the strongest mutant-like phenotype and the flowers return to a wild-type state.
- Correlate the phenotype you observed at anthesis with the stage at which the transgene was activated (Note 24).
- Compile a stage-dependent phenotyping series to compare with expression profiling data.
- Grow plants so that they bolt in a relatively synchronous manner (Note 18).
- Controls
We outline the controls below that can be used for each perturbation strategy. In addition, we outline controls that are required for the inducible systems themselves. These latter controls are extremely important, as we have previously characterized the effects of these inducible systems on the transcriptome of A. thaliana (O'Maoileidigh et al., 2015).
Transcriptional effector-mediated perturbations: Essential sequences in the transcriptional effector domain of choice can be mutated to produce a functionally inactive transcriptional effector domain [e.g., mSRDX (Hiratsu et al., 2004) or mVP16 (Cress and Triezenberg, 1991)]. These inactive domains can be fused to the factor of interest and expressed in an identical way to the primary experimental line. The protein-mEAR fusion should behave as a gain-of-function line. Therefore, it may also be desirable to generate an equivalent gain-of-function line that lacks an EAR motif as a control.
amiRNA-mediated perturbation: We have previously described a CONTROL-amiRNA sequence that is not predicted to target any annotated A. thaliana transcript (O'Maoileidigh et al., 2015). Therefore, this CONTROL-amiRNA can be expressed in an identical manner to the primary experimental line to control for expression or phenotypic artifacts. An additional control includes generating an amiRNA-resistant version of the factor of interest by introducing synonymous mutations in the amiRNA target site of the corresponding gene. This would require the generation of an equivalent mutant plant whose phenotype is restored by an amiRNA-resistant factor.
AlcR and GR-LhG4 transcription factors: These chemically responsive transcription factors have been shown to influence the expression of the A. thaliana transcriptome (O'Maoileidigh et al., 2015). Therefore, an essential control is to transform plants with T-DNAs that contain the AlcApro/AlcR or OPpro/GR-LhG4 cassettes and identify plants that express the transcription factors to a similar level to the primary experimental line.
Treatment controls: All treatment controls should be partnered with a “mock” treatment. This mock treatment solution should consist of an identical mix of the solvents and surfactants used but lacking the active ingredient. An additional control includes the treatment of a wild-type line, or an equivalent line, with the active solution.
Notes
- For example, the GR domain was fused to the C-terminus of AG since the DNA binding domain of AG is present at the N-terminus (Ito et al., 2004). It is possible that several versions with the tag of interest in different positions may need to be made.
- Often short linkers of approximately 6-10 amino acids are placed between the protein of interest and the domain being fused to it (Sabourin et al., 2007). This may reduce the likelihood of unwanted negative interactions. A repeat of nine alanine amino acids or a mix of glycine and alanine are commonly used in plants (Tian et al., 2004; Heisler et al., 2005).
- Often the CMV 35S promoter is used (Benfey and Chua, 1990), however, it may not drive expression sufficiently in the tissues of interest. The promoter of UBIQUITIN10 is frequently used as an alternative for constitutive expression (Grefen et al., 2010). Promoters that drive expression only in certain tissues can also be used. This latter option may be desirable, especially if the inducible transgene is being expressed in its mutant background, as it may reduce the occurrence of false positives.
- The promoters upstream of the AlcR and GR-LhG4 chimeric transcription factors can be altered to suit the experimental design.
- Mutant plants can be useful in this case in order to show that the fusion protein can restore wild-type activity, as in the case of 35Spro:AG-GR ag-1 plants (Ito et al., 2004). The use of wild-type plants to identify a gain-of-function phenotype is also possible, however, it may be more difficult to interpret.
- The concentration of dexamethasone or DHT to use may need to be optimized. As little as 50 nM DHT has been used to activate gene expression (Sun et al., 2009). Ten μM dexamethasone is commonly used, however, the use of a lower concentration may be desirable (Yamaguchi et al., 2015). Silwett L-77 should be used at a concentration of 0.015 % (v/v) to improve the uptake of the chemical. We have used ethanol vapor to activate the AlcApro/AlcR system (O’Maoileidigh et al., 2013; Wuest et al., 2012). Briefly, we enclosed plants in sealed containers (18 cm x 32 cm x 50 cm) with two 50 ml tubes containing 10 ml 100% ethanol for a range of times (O’Maoileidigh et al., 2013; Wuest et al., 2012).
- This was elegantly shown by Ito et al. (2007), who used this strategy to dissect the stage-specific activities of AG. Flowers of 35Spro:AG-GR ag-1 plants that were treated with dexamethasone at early stages of development produced mature flowers containing carpels and stamens whereas flowers treated at later contained carpelloid sepals and stamenoid petals (Ito et al., 2004).
- If using the GR/AR fusion technique, the mRNA levels of the fusion gene can be determined. If using a two-component inducible system, the mRNA levels of the gene of interest can be determined after treatment with the inducer. Alternatively, the mRNA levels of the AlcR/GR-LhG4 genes can be determined, which should correlate with the expression output of the gene of interest.
- If selecting plants based on expression levels, it may be desirable to identify plants with strong, intermediate and weak expression levels of the transgene as the effects of extremely high expression can have negative consequences for downstream applications.
- The protein of interest does not necessarily have to be a transcription factor, however, it must be localized in proximity to DNA.
- The chimeric protein may not function as expected and the screening of inducible versions can be laborious. Therefore, the production of a constitutively expressed version is advised. Promoters that drive expression only in certain tissues can also be used.
- In order to interpret the phenotype produced, it may be necessary to identify null mutants or gain-of-function mutants of the same gene.
- Multiple amiRNAs may need to be screened, therefore, the expression of these amiRNAs from a constitutive promoter to select a functional amiRNA is advised.
- The region cloned to generate a dsRNA construct should not be homologous to other annotated genes.
- We prefer to select amiRNAs from the WMD3 platform (wmd3.weigelworld.org) so that the gene body is tiled with different amiRNAs. This is because the secondary structure of the target mRNA may interfere with amiRNA function.
- Strong expression of the AlcR or GR-LhG4 transcription factors exacerbate non-specific effects. Therefore, a balance between the level of the knockdown and the expression of the chemically-responsive transcription factor should be reached.
- We rotate our plants in the growth chambers to minimize effects of differing temperature and light gradients that might be present.
- The depletion and recovery of the mRNA of the target gene in response to the activation of the RNAi/amiRNA construct will dictate the time at which tissue will be harvested. The optimal time to collect tissue would be when the mRNA levels of the target gene are lowest, however, protein abundance of the target may also need to be measured.
- There are dexamethasone and DHT-responsive FISs available. The AlcApro/AlcR two-component system is compatible with either, however, the OPpro/GR-LhG4 system is compatible only with the DHT-responsive FIS.
- There are versions of the FIS that are resistant to glufosinate (‘BASTA’) and kanamycin treatments, respectively (O'Maoileidigh et al., 2015). Therefore, these plants can be transformed with a T-DNA containing the inducible transgene of interest with a compatible selection marker.
- If the recovery of the mRNA levels of the targeted factor is rapid, then multiple or continuous treatments may be necessary prior to see strong mutant phenotypes.
- The AP1pro:AP1-GR ap1 cal FIS can be activated by treating the inflorescences with a solution containing 10 μM dexamethasone, 0.015% (v/v) Silwett L-77. The AP1pro:AP1-AR ap1 cal FIS can be activated by treating the inflorescences with a solution containing from 500 μM DHT (higher concentrations can be used), 0.015% (v/v) Silwett L-77.
- The flowers at early stages should be scraped from the surface of the inflorescence with sharp forceps rather than harvesting the entire inflorescence (O'Maoileidigh and Wellmer, 2014).
- Smyth et al. (1990) characterized the development of A. thaliana flowers in detail, which includes the approximate timings of each developmental stage. The length of these stages can be correlated with the perturbation series generated using the inducible perturbation line.
Recipes
- 10 mM dexamethasone stock solution
Resuspend dexamethasone powder in 100% EtOH to make a 10 mM stock
Stored at -20 °C - 100 mM 5α-Androstan-17β-ol-3-one (DHT) stock solution
Resuspend DHT in 100% EtOH to make a 100 mM stock
Stored at -20 °C - GR activation solution
10 μM dexamethasone
0.015% (v/v) Silwet L-77
0.1% (v/v) ethanol - AR activation solution
500 μM DHT
0.015% (v/v) Silwet L-77
0.5% (v/v) ethanol
Acknowledgments
This work was supported by grants from Science Foundation Ireland to F. W. and E. G. This protocol was established using the knowledge and reagents from several previous studies (O'Maoileidigh et al., 2015; O'Maoileidigh and Wellmer, 2014; O'Maoileidigh and Wellmer, 2014; O'Maoileidigh et al., 2013). We thank Dr. Jeff Long for the gifts of the OPpro-pBJ36 and 35Spro:GR-LhG4-pML-BART plasmids. We thank the three anonymous reviewers for their helpful comments.
References
- Benfey, P. N. and Chua, N. H. (1990). The cauliflower mosaic virus 35S promoter: Combinatorial regulation of transcription in plants. Science 250(4983): 959-966.
- Birnbaum, K., Shasha, D. E., Wang, J. Y., Jung, J. W., Lambert, G. M., Galbraith, D. W. and Benfey, P. N. (2003). A gene expression map of the Arabidopsis root. Science 302(5652): 1956-1960.
- Bowman, J. L., Smyth, D. R. and Meyerowitz, E. M. (1989). Genes directing flower development in Arabidopsis. Plant Cell 1(1): 37-52.
- Clough, S. J. and Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6): 735-743.
- Cousens, D. J., Greaves, R., Goding, C. R. and O'Hare, P. (1989). The C-terminal 79 amino acids of the herpes simplex virus regulatory protein, Vmw65, efficiently activate transcription in yeast and mammalian cells in chimeric DNA-binding proteins. EMBO J 8(8): 2337-2342.
- Craft, J., Samalova, M., Baroux, C., Townley, H., Martinez, A., Jepson, I., Tsiantis, M. and Moore, I. (2005). New pOp/LhG4 vectors for stringent glucocorticoid-dependent transgene expression in Arabidopsis. Plant J 41(6): 899-918.
- Cress, W. D. and Triezenberg, S. J. (1991). Critical structural elements of the VP16 transcriptional activation domain. Science 251(4989): 87-90.
- Deveaux, Y., Peaucelle, A., Roberts, G. R., Coen, E., Simon, R., Mizukami, Y., Traas, J., Murray, J. A., Doonan, J. H. and Laufs, P. (2003). The ethanol switch: a tool for tissue-specific gene induction during plant development. Plant J 36(6): 918-930.
- Eamens, A. L. and Waterhouse, P. M. (2011). Vectors and methods for hairpin RNA and artificial microRNA-mediated gene silencing in plants. Methods Mol Biol 701: 179-197.
- Gomez-Mena, C., de Folter, S., Costa, M. M., Angenent, G. C. and Sablowski, R. (2005). Transcriptional program controlled by the floral homeotic gene AGAMOUS during early organogenesis. Development 132(3): 429-438.
- Grefen, C., Donald, N., Hashimoto, K., Kudla, J., Schumacher, K. and Blatt, M. R. (2010). A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J 64(2): 355-365.
- Heisler, M. G., Ohno, C., Das, P., Sieber, P., Reddy, G. V., Long, J. A. and Meyerowitz, E. M. (2005). Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15(21): 1899-1911.
- Hiratsu, K., Mitsuda, N., Matsui, K. and Ohme-Takagi, M. (2004). Identification of the minimal repression domain of SUPERMAN shows that the DLELRL hexapeptide is both necessary and sufficient for repression of transcription in Arabidopsis. Biochem Biophys Res Commun 321(1): 172-178.
- Hiratsu, K., Ohta, M., Matsui, K. and Ohme-Takagi, M. (2002). The SUPERMAN protein is an active repressor whose carboxy-terminal repression domain is required for the development of normal flowers. FEBS Lett 514(2-3): 351-354.
- Ikeda, M. and Ohme-Takagi, M. (2009). A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol 50(5): 970-975.
- Ikeda, M., Mitsuda, N. and Ohme-Takagi, M. (2009). Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell 21(11): 3493-3505.
- Ito, T., Ng, K. H., Lim, T. S., Yu, H. and Meyerowitz, E. M. (2007). The homeotic protein AGAMOUS controls late stamen development by regulating a jasmonate biosynthetic gene in Arabidopsis. Plant Cell 19(11): 3516-3529.
- Ito, T., Wellmer, F., Yu, H., Das, P., Ito, N., Alves-Ferreira, M., Riechmann, J. L. and Meyerowitz, E. M. (2004). The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 430(6997): 356-360.
- Kaufmann, K., Wellmer, F., Muino, J. M., Ferrier, T., Wuest, S. E., Kumar, V., Serrano-Mislata, A., Madueno, F., Krajewski, P., Meyerowitz, E. M., Angenent, G. C. and Riechmann, J. L. (2010). Orchestration of floral initiation by APETALA1. Science 328(5974): 85-89.
- Leibfried, A., To, J. P., Busch, W., Stehling, S., Kehle, A., Demar, M., Kieber, J. J. and Lohmann, J. U. (2005). WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438(7071): 1172-1175.
- Mantegazza, O., Gregis, V., Chiara, M., Selva, C., Leo, G., Horner, D. S. and Kater, M. M. (2014). Gene coexpression patterns during early development of the native Arabidopsis reproductive meristem: novel candidate developmental regulators and patterns of functional redundancy. Plant J 79(5): 861-877.
- Miki, B. and McHugh, S. (2004). Selectable marker genes in transgenic plants: applications, alternatives and biosafety. J Biotechnol 107(3): 193-232.
- Mitsuda, N., Hiratsu, K., Todaka, D., Nakashima, K., Yamaguchi-Shinozaki, K. and Ohme-Takagi, M. (2006). Efficient production of male and female sterile plants by expression of a chimeric repressor in Arabidopsis and rice. Plant Biotechnol J 4(3): 325-332.
- Mitsuda, N., Matsui, K., Ikeda, M., Nakata, M., Oshima, Y., Nagatoshi, Y. and Ohme-Takagi, M. (2011). CRES-T, an effective gene silencing system utilizing chimeric repressors. Methods Mol Biol 754: 87-105.
- O'Maoileidigh, D. S. and Wellmer, F. (2014). A floral induction system for the study of early Arabidopsis flower development. Methods Mol Biol 1110: 307-314.
- O’Maoileidigh, D. S., Wuest, S. E., Rae, L., Raganelli, A., Ryan, P. T., Kwasniewska, K., Das, P., Lohan, A. J., Loftus, B., Graciet, E. and Wellmer, F. (2013). Control of reproductive floral organ identity specification in Arabidopsis by the C function regulator AGAMOUS. Plant Cell 25(7): 2482-2503.
- O'Maoileidigh, D. S., Thomson, B., Raganelli, A., Wuest, S. E., Ryan, P. T., Kwasniewska, K., Carles, C. C., Graciet, E. and Wellmer, F. (2015). Gene network analysis of Arabidopsis thaliana flower development through dynamic gene perturbations. Plant J 83(2): 344-358.
- Ossowski, S., Schwab, R. and Weigel, D. (2008). Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J 53(4): 674-690.
- Parcy, F., Nilsson, O., Busch, M. A., Lee, I. and Weigel, D. (1998). A genetic framework for floral patterning. Nature 395(6702): 561-566.
- Roslan, H. A., Salter, M. G., Wood, C. D., White, M. R., Croft, K. P., Robson, F., Coupland, G., Doonan, J., Laufs, P., Tomsett, A. B. and Caddick, M. X. (2001). Characterization of the ethanol-inducible alc gene-expression system in Arabidopsis thaliana. Plant J 28(2): 225-235.
- Ryan, P. T., O'Maoileidigh, D. S., Drost, H. G., Kwasniewska, K., Gabel, A., Grosse, I., Graciet, E., Quint, M. and Wellmer, F. (2015). Patterns of gene expression during Arabidopsis flower development from the time of initiation to maturation. BMC Genomics 16: 488.
- Sabourin, M., Tuzon, C. T., Fisher, T. S. and Zakian, V. A. (2007). A flexible protein linker improves the function of epitope-tagged proteins in Saccharomyces cerevisiae. Yeast 24(1): 39-45.
- Schwab, R., Ossowski, S., Riester, M., Warthmann, N. and Weigel, D. (2006). Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18(5): 1121-1133.
- Smith, J., Morgan, J. R., Zottoli, S. J., Smith, P. J., Buxbaum, J. D. and Bloom, O. E. (2011). Regeneration in the era of functional genomics and gene network analysis. Biol Bull 221(1): 18-34.
- Smyth, D. R., Bowman, J. L. and Meyerowitz, E. M. (1990). Early flower development in Arabidopsis. Plant Cell 2(8): 755-767.
- Sun, B., Xu, Y., Ng, K. H. and Ito, T. (2009). A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes Dev 23(15): 1791-1804.
- Tian, G. W., Mohanty, A., Chary, S. N., Li, S., Paap, B., Drakakaki, G., Kopec, C. D., Li, J., Ehrhardt, D., Jackson, D., Rhee, S. Y., Raikhel, N. V. and Citovsky, V. (2004). High-throughput fluorescent tagging of full-length Arabidopsis gene products in planta. Plant Physiol 135(1): 25-38.
- Triezenberg, S. J., Kingsbury, R. C. and McKnight, S. L. (1988). Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev 2(6): 718-729.
- Wellmer, F., Alves-Ferreira, M., Dubois, A., Riechmann, J. L. and Meyerowitz, E. M. (2006). Genome-wide analysis of gene expression during early Arabidopsis flower development. PLoS Genet 2(7): e117.
- Wellmer, F., Riechmann, J. L., Alves-Ferreira, M. and Meyerowitz, E. M. (2004). Genome-wide analysis of spatial gene expression in Arabidopsis flowers. Plant Cell 16(5): 1314-1326.
- Wuest, S. E. and Grossniklaus, U. (2014). Laser-assisted microdissection applied to floral tissues. Methods Mol Biol 1110: 329-344.
- Wuest, S. E., O'Maoileidigh, D. S., Rae, L., Kwasniewska, K., Raganelli, A., Hanczaryk, K., Lohan, A. J., Loftus, B., Graciet, E. and Wellmer, F. (2012). Molecular basis for the specification of floral organs by APETALA3 and PISTILLATA. Proc Natl Acad Sci U S A 109(33): 13452-13457.
- Yamaguchi, N., Winter, C. M., Wellmer, F. and Wagner, D. (2015). Identification of direct targets of plant transcription factors using the GR fusion technique. Methods Mol Biol 1284: 123-138.
- Yanofsky, M. F., Ma, H., Bowman, J. L., Drews, G. N., Feldmann, K. A. and Meyerowitz, E. M. (1990). The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346(6279): 35-39.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ó’Maoiléidigh, D. S., Graciet, E. and Wellmer, F. (2016). Strategies for Performing Dynamic Gene Perturbation Experiments in Flowers. Bio-protocol 6(7): e1774. DOI: 10.21769/BioProtoc.1774.
Category
Plant Science > Plant developmental biology > General
Plant Science > Plant molecular biology > DNA > Mutagenesis
Molecular Biology > DNA > Mutagenesis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










