- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Mitotic and Meiotic Metaphase Chromosomes from Young Leaves and Flower Buds of Coccinia grandis
Published: Vol 6, Iss 7, Apr 5, 2016 DOI: 10.21769/BioProtoc.1771 Views: 16314
Reviewed by: Samik BhattacharyaXinyan ZhangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions
Yuta Kato [...] Mitsutaka Taniguchi
Aug 5, 2025 2323 Views

Live-Cell Monitoring of Piecemeal Chloroplast Autophagy
Masanori Izumi [...] Shinya Hagihara
Nov 5, 2025 1691 Views

Chloroplast Movement Imaging Under Different Light Regimes With a Hyperspectral Camera
Paweł Hermanowicz [...] Justyna Łabuz
Dec 20, 2025 756 Views
Abstract
Somatic chromosomes are usually studied from the root tip cells of the plants for cytological investigations. In dioecious plant species like Coccinia grandis, it is very difficult to get meristematic root tip cells from the mature plants of the respective sex forms. In this report, young leaves of the respective sexual phenotypes were used as tissue samples for mitotic chromosome analysis. For meiotic preparation, flower buds of appropriate size were selected for chromosomal studies. Following protocols could be effectively used for routine chromosome preparations in other plant species as well.
Keywords: Sex chromosomesMaterials and Reagents
- Blotting papers
- Glass slides and cover-slips
- Specimen tube
- 1st, 2nd and 3rd leaf from the shoot apical region for mitotic study
Note: Young leaves can be harvested from seedlings as well as from mature plants. - Flower buds of 7 and 8 stages for meiotic study
Note: Assigned according to the length of flower buds. - Saturated solution of para-dichlorobenzene (Sigma-Aldrich, catalog number: D56829 )
Note: It is also named “1,4-Dichlorobenzene” on Sigma-Aldrich website. - Glacial acetic acid (SRL, catalog number: 85801 )
- Hydrochloric Acid (HCl)
- Orcein (Sigma-Aldrich, catalog number: O7380 )
- Carmine (Sigma-Aldrich, catalog number: C1022 )
- Absolute ethanol (99%) (Merck Millipore Corporation, catalog number: 100983 )
- 2% w/v aceto-orcein stain (see Recipes)
- 1% w/v aceto-carmine stain (see Recipes)
Equipment
- Compound microscope (ZEISS, model: Primo-star )
- Refrigerator (household refrigerator having a freezer compartment with sub-zero temperature)
Procedure
- Mitotic metaphase chromosome study (Video 1)Video 1. The MS-preparation of mitotic and meiotic metaphase chromosomes
- Selected young leaves (Figure 1A) from healthy plants grown in experimental garden were collected at 9-10 AM for pre-treatment.
- Only apical region of the young leaves was cut (Figure 1B) and kept in a specimen tube containing cold saturated solution of para-dichlorobenzene for 5 min at 0 °C (in freezer compartment of normal household refrigerator) followed by 6 h pre-treatment at 10-12 °C (in the freeze compartment of normal household refrigerator).
- Pre-treated leaf tips were then thoroughly washed with distilled water and fixed in acetic acid: ethanol mixture (1:3) for overnight at room temperature.
- Leaf tips were transferred in 45% acetic acid for 15 min and then washed with distilled water, followed by hydrolysis with 5 N HCl for 20 min in refrigerator.
- Leaf tips were again thoroughly washed with distilled water and then immersed in 45% acetic acid for 5 min followed by staining with 2% aceto-orcein for overnight.
- Leaf tips were then squashed and mounted in 45% acetic acid (by putting a coverslip on leaf tip and applying gradual pressure with thumb in order to spread out the cells) and observed under a microscope.
- Photomicrographs were taken with Zeiss primo-star Microscope (any compound microscope can be used having 40x or 100x objective with oil immersion) and suitably enlarged (Figure1 C-E).
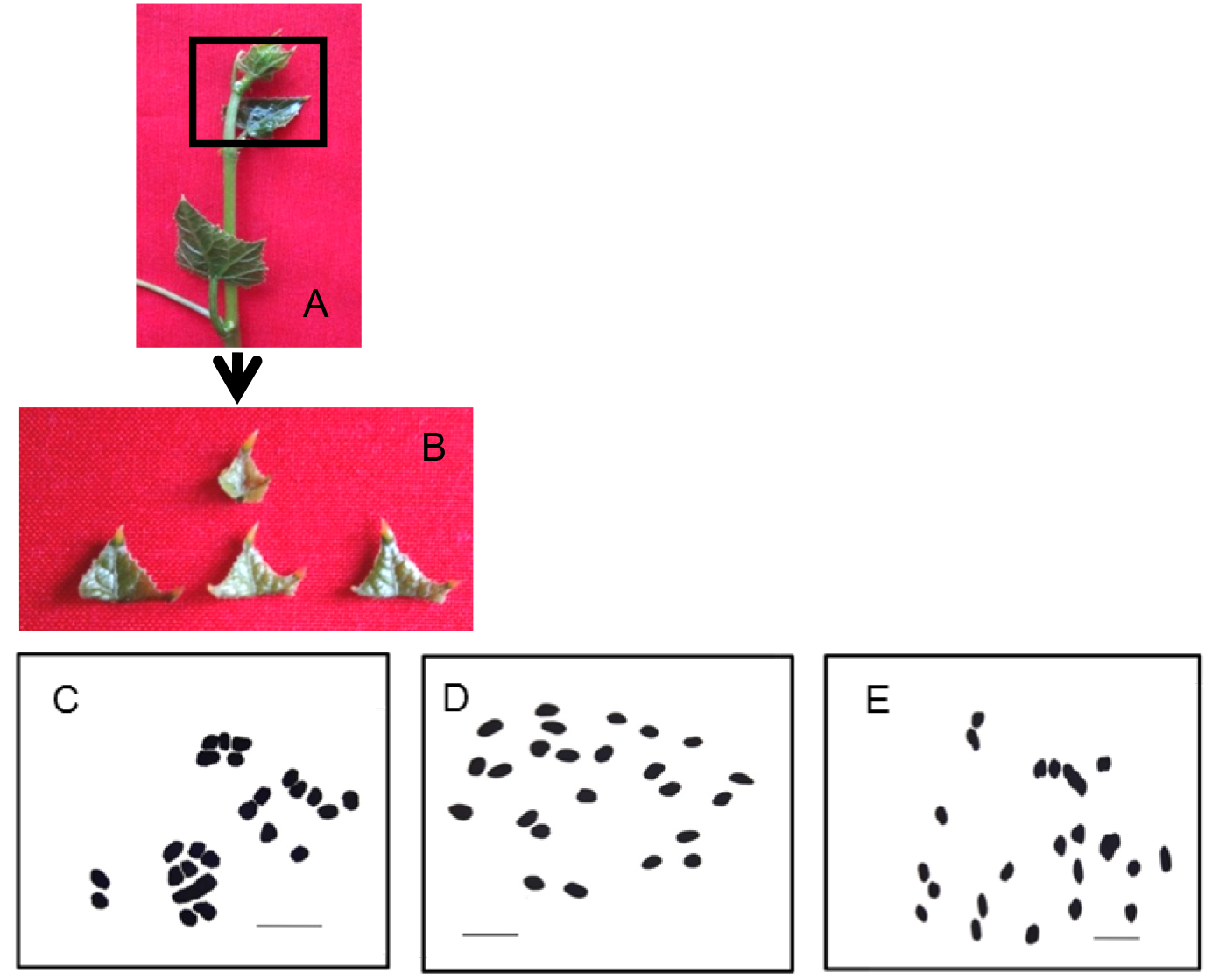
Figure 1. A-B. Tissue samples for mitotic chromosome study in Coccinia grandis; A. 1st, 2nd and 3rd leaves were selected for pre-treatment; B. Only apical regions of young leaves were selected and the basal regions were discarded; C. Mitotic metaphase male plant; D. Female plant; E. Gynomonoecious plant. Scale bar=5 µm
- Selected young leaves (Figure 1A) from healthy plants grown in experimental garden were collected at 9-10 AM for pre-treatment.
- Meiotic metaphase chromosome study
- Flower buds of seventh and eighth developmental stages (Figure 2A-B) were collected directly from the field at 11-11:30 AM during April-May.
- Sepals and petals were removed from the selected flower buds and the anthers were kept in 45% acetic acid for 5 min.
- A part of the fused anthers was smeared in a drop of 2% aceto-carmine stain.
- After 10 min, the prepared slide was slightly warmed by passing over a flame.
- After 30 min, the stained cells were washed by adding drops of 45% acetic acid on one side of the cover glass and by drawing the access fluid from the opposite side of the cover glass with the help of a strip of blotting paper; this step is crucial for clearing the cytoplasmic background of the stained pollen mother cells.
- Finally, the prepared slide is wrapped in a fold of blotting paper to remove access of 45% acetic acid leaving only a thin film of fluid in between slide and cover-slip.
- Photomicrographs were taken with Leica Microscope and suitably enlarged (Figure 2C-D).
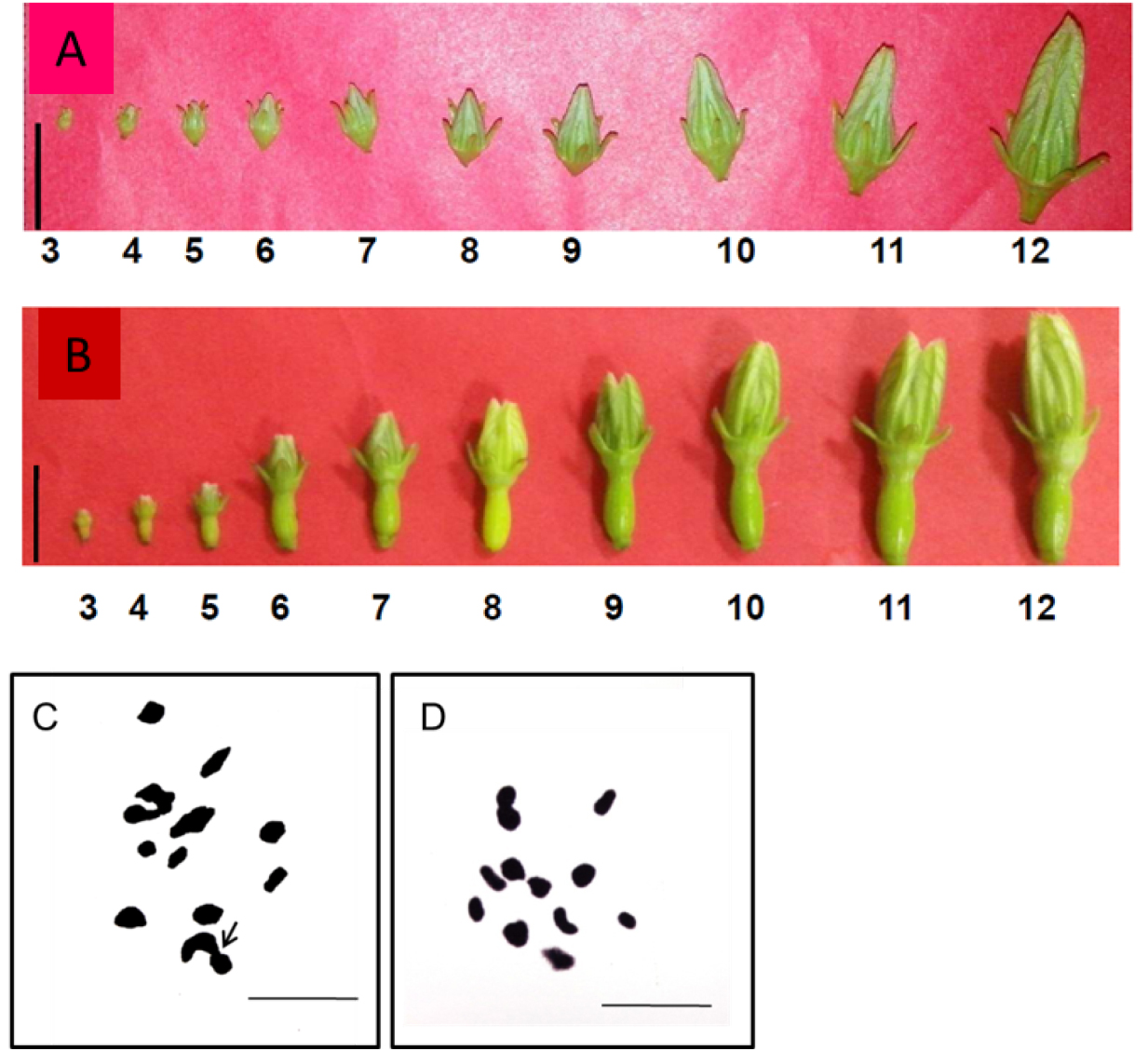
Figure 2. A. Stages of male flower buds of Coccinia grandis for meiotic preparation; B. Gynomonoecious hermaphrodite flower buds; C. Meiotic metaphase chromosome of male (Arrow indicates end to end pairing of X and Y chromosomes.); D. Gynomonoecious plant. Scale bar=1 cm for A and B, 5 µm for C and D,
- Flower buds of seventh and eighth developmental stages (Figure 2A-B) were collected directly from the field at 11-11:30 AM during April-May.
Notes
Material should be collected on a bright sunny day to ensure proper mitotic stage development. Cloudy or rainy day should be avoided for material collection.
Recipes
- 2% w/v aceto-orcein stain
Add orcein powder to boiling 45% glacial acetic acid, cool rapidly, and then filter into a dark glass bottle using any laboratory grade filter paper. - 1% w/v aceto-carmine stain
Add carmine powder to boiling 45% glacial acetic acid, cool rapidly, and then filter into a dark glass bottle using any laboratory grade filter paper.
Acknowledgments
Financial support from Department of Biotechnology (DBT), Govt. of India is thankfully acknowledged. Core support from IISER Pune is also acknowledged.
References
- Bhowmick, B. K., Jha, T. B. and Jha, S. (2012). Chromosome analysis in the dioecious cucurbit Coccinia grandis (L.) Voigt. Chromosome Science 15: 9-15.
- Ghadge, A. G., Karmakar, K., Devani, R. S., Banerjee, J., Mohanasundaram, B., Sinha, R. K., Sinha, S. and Banerjee, A. K. (2014). Flower development, pollen fertility and sex expression analyses of three sexual phenotypes of Coccinia grandis. BMC Plant Biol 14: 325.
- Kumar, L. S. and Vishveshwaraiah, S. (1952). Sex mechanism in Coccinia indica wight and arn. Nature 170(4321): 330-331.
- Roy, R. P. and Roy, P. M. (1971). Mechanism of sex determination in Cocciniaindica. J Indian Bot Soc 50A: 391-400.
- Sousa, A., Fuchs, J. and Renner, S. S. (2013). Molecular cytogenetics (FISH, GISH) of Coccinia grandis: a ca. 3 myr-old species of cucurbitaceae with the largest Y/autosome divergence in flowering plants. Cytogenet Genome Res 139(2): 107-118.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sinha, S., Karmakar, K., Devani, R. S., Banerjee, J., Sinha, R. K. and Banerjee, A. K. (2016). Preparation of Mitotic and Meiotic Metaphase Chromosomes from Young Leaves and Flower Buds of Coccinia grandis. Bio-protocol 6(7): e1771. DOI: 10.21769/BioProtoc.1771.
Category
Plant Science > Plant cell biology > Cell imaging
Cell Biology > Cell structure > Chromosome
Molecular Biology > DNA > DNA structure
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
















