- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Detection of Nitric Oxide and Determination of Nitrite Concentrations in Arabidopsis thaliana and Azospirilum brasilense
(*contributed equally to this work) Published: Vol 6, Iss 6, Mar 20, 2016 DOI: 10.21769/BioProtoc.1765 Views: 21269
Reviewed by: Tie LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visualization of Nitric Oxide, Measurement of Nitrosothiols Content, Activity of NOS and NR in Wheat Seedlings
Sandeep B. Adavi [...] Shailendra K. Jha
Oct 20, 2019 6354 Views

A Quick Method to Quantify Iron in Arabidopsis Seedlings
Chandan Kumar Gautam [...] Wolfgang Schmidt
Mar 5, 2022 3932 Views

CAPS-Based SNP Genotyping for Nitrogen-Response Phenotypes in Maize Hybrids
Jannis Jacobs [...] Peter K. Lundquist
Dec 20, 2025 550 Views
Abstract
There is now general agreement that nitric oxide (NO) is an important and almost ubiquitous signal in plants. Nevertheless, there are still many controversial observations and differing opinions on the importance and functions of NO in plants. Partly, this may be due to the difficulties in detecting and quantifying NO. Here, we summarize protocols for detecting NO and quantifying nitrite concentration in Arabidopsis seedlings. We also present a method to measure NO in biofilms formed by the plant growth promoting rhizobacteria Azospirillum brasilense (A. brasilense). NO in oxygen-containing aqueous solutions has a short half-life that is often attributed to a rapid oxidation to nitrite. Here we detail the use of the fluorescent probe DAF-FM DA and the electrochemical method for directly detecting and quantifying NO, respectively, and the Griess reagent to indirectly detect NO through its oxidized nitrite form. These protocols could be useful in a variety of cell types and plant tissues, as well as for microorganisms.
Keywords: Nitric oxide
Part I. In vitro determination of nitrite concentration
Materials and Reagents
- Square Petri dishes (Deltalab, catalog number: 200204 )
- Multi-well plates (96 well) (Deltalab, catalog number: 900010 )
- 10-day-old Arabidopsis ecotype Columbia Col-0
- Murashige and Skoog Basal Salt Mixture (MS) (Sigma-Aldrich, catalog number: M5524 )
- Sodium choride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- Sulfanilamide (Sigma-Aldrich, catalog number: S9251 )
Note: The working solution is 1% (w/v) Sulfanilamide in 5% (v/v) phosphoric acid. Store at 4 ºC in the dark. - N-(1-Naphthyl)ethylenediamine dihydrochloride (NED) (Sigma-Aldrich, catalog number: 33461 )
Note: The working solution is 0.1% (w/v) NED in H2O. Store at 4 ºC in the dark. - Standard nitrite solution (Sigma-Aldrich, catalog number: 237213 )
Note: The working solution is 100 µM sodium nitrite in Milli Q water. - Sodium phosphate dibasic (Sigma-Aldrich, catalog number: S0876 )
- Sodium phosphate monobasic (Sigma-Aldrich, catalog number: 0751 )
- Buffer A (100 mM phosphate buffer, pH 7.4) (see Recipes)
Equipment
- Centrifuge (Thermo Fisher Scientific, model: Sorvall Legend Micro 17R )
- Elisa plate reader (Metrolab 980 microplate reader)
Procedure
- Grow Arabidopsis in Petri dishes containing ½ strength MS medium for 5 d and then transfer to treatment solution (100 mM NaCl) for 5 d or more (step 1, Figure 1).
- Grind 100 mg of seedlings or dissect the plant into root and shoot to a powder under liquid nitrogen in a mortar. Add 300 µl of 100 mM sodium phosphate (pH 7.4) to the samples [step 2(a), Figure 1].
- Centrifuge samples at 10,000 x g for 15 min at 4 °C [step 2(b), Figure 1b].
- Use the supernatant for nitrite and protein quantification: For nitrite quantification, load 50 µl of supernatant in a well of the Elisa plate in triplicate [step 2(c), Figure 1]. For protein quantification, perform the Bradford (1976) assay with 1 or 2 µl of supernatant in triplicate.
- For the nitrite Standard, prepare 1 ml of a 100 μM nitrite solution [step 2(c), Figure 1]. Dispense 50 μl of the Buffer A into the wells in rows B-H. Add 100 μl of the 100 μM nitrite solution to the remaining 3 wells in the first row. Immediately perform 6 serial two-fold dilutions (50 μl/well) in triplicate down the plate to generate the nitrite Standard reference curve (100, 50, 25, 12.5, 6.25, 3.13 and 1.56 μM). Do not add any nitrite solution to the last set of wells (0 μM) as this will serve as the blank measurement.
- Allow the Sulfanilamide Solution and NED Solution to equilibrate to room temperature (15-30 min). Dispense 50 μl of the Sulfanilamide Solution to all experimental samples and wells containing the dilution series for the nitrite Standard reference curve [step 2(c), Figure 1].
- Incubate for 5-10 min at room temperature, protected from light.
- Dispense 50 μl of the NED Solution to all wells (step 3, Figure 1). An appropriate control assay without NED solution is necessary to detect any interference due to sample.
- Incubate at room temperature for 5-10 min, protected from light. A purple/magenta color will begin to form, if the Griess reaction has occurred (step 3, Figure 1).
- Measure absorbance in a plate reader with a filter between 520 nm and 550 nm. A single measurement for each well is sufficient. The measurement should be done within 30 min after step 9 (step 3, Figure 1), because the color may fade after this time.
- Use the standard curve to calculate the nitrite concentration in the samples. Calculate the concentration as nitrite per µg of protein. Use the equation (step 4, Figure 1) for nitrite calculations. The “y” value is the absorbance detected for each sample and the “x” value, is the nitrite concentration.
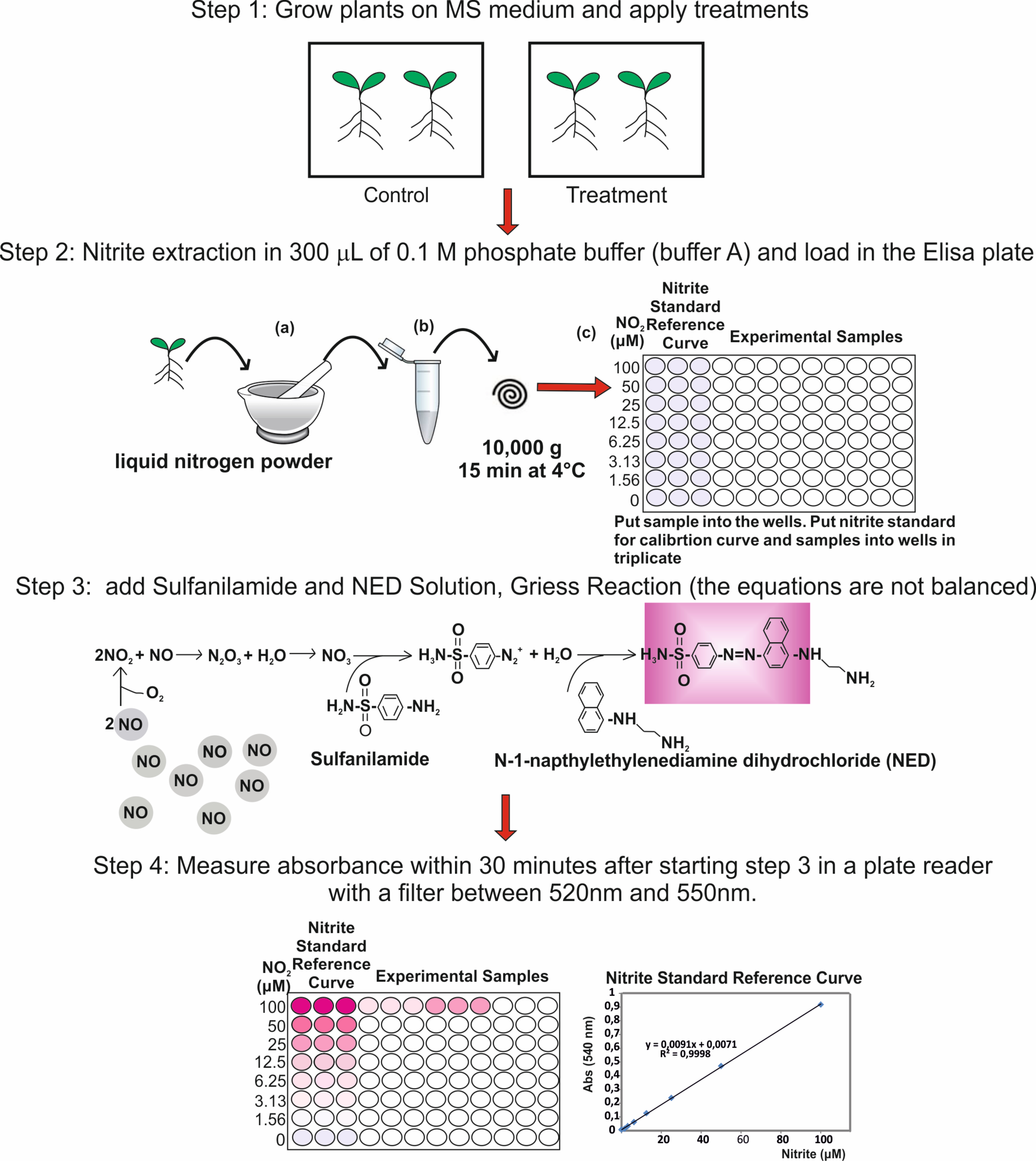
Figure 1. Nitrite determination by Griess assay in Arabidopsis seedlings
Recipes
- Buffer A (100 mM phosphate buffer, pH 7.4)
77.4 mM Sodium phosphate dibasic
22.6 mM Sodium phosphate monobasic
Store at room temperature
Part II. In vivo detection of NO
Method 1. Electrochemical detection of NO
Materials and Reagents
- Multi-well plates (24 wells flat bottom) (Sigma-Aldrich, Corning® Costar®, catalog number: CLS3527 )
- 20 ml borosilicate glass vial (Thermo Fisher Scientific, catalog number: 033377 )
- Azospirillum brasilense Sp245 strain
- Standard nitrite solution (Sigma-Aldrich, catalog number: 237213 )
Note: The working solution is 100 µM sodium nitrite in Milli Q water. Prepare fresh for each use. - Potassium iodide (Sigma-Aldrich, catalog number: 746428 )
- Sulfuric acid (Merck Millipore Corporation, catalog number: 100732 )
- DL-Malic acid (Sigma-Aldrich, catalog number: 240176 )
- Potassium phosphate dibasic (K2HPO4) (Sigma-Aldrich, catalog number: P3786 )
- Magnesium sulfate heptahydrate (MgSO4.7H2O) (Sigma-Aldrich, catalog number: 230391 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Calcium chloride hydrate (CaCl2.H2O) (Sigma-Aldrich, catalog number: 202940 )
- Ethylenediaminetetraacetic acid ferric sodium salt (Fe-EDTA) (Sigma-Aldrich, catalog number: E6760 )
- Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: P1767 )
- Potassium nitrate (KNO3) (Sigma-Aldrich, catalog number: P8291 )
- Sodium molybdate dehydrate (NaMoO4.2H2O) (Sigma-Aldrich, catalog number: 331058 )
- Manganese(II) sulfate monohydrate (MnSO4) (Sigma-Aldrich, catalog number: M7634 )
- Boric acid (H3BO3) (Sigma-Aldrich, catalog number: B6768 )
- Copper(II) sulfate pentahydrate (CuSO4.5H2O) (Sigma-Aldrich, catalog number: C8027 )
- Zinc sulfate heptahydrate (ZnSO4.7H2O) (Sigma-Aldrich, catalog number: Z0251 )
- Phosphate buffered saline, pH 7.4 (Sigma-Aldrich, catalog number: P4417 )
- Calibration solution (see Recipes)
Note: Prepare fresh for use. - Buffer B (see Recipes)
- NFb-malic medium (see Recipes)
Equipment
- Nitric Oxide Measuring System (NOMS) (e.g. Innovative Instruments Inc., model: inNO-T-II System )
- NO-specific sensor (e.g. Innovative Instruments Inc., model: amiNO-2000 )
- Sensoready (Innovative Instrument) device
Sensor calibration- Before calibration the sensor should be polarized for a few hours (preferably overnight) by immersing it in calibration solution or Milli Q water and connect to the Sensoready device.
- Turn on PC and open NOMS software.
- The sensor is calibrated by a chemical reaction for NO production based on the conversion of nitrite to NO in acidic solution in the presence of iodide ion. The reaction has a molar ratio 1:1, meaning that the amount of NO produced equals the amount of nitrite added. In this protocol we used the term “NO/nitrite” concentration to unify both.
Note: Other methods such as using the NO donor (±)-S-Nitroso-N-acetylpenicillamine (SNAP) and NO saturated solutions can be assayed (Allen et al., 2003). - Immerse the tip of the sensor in the calibration solution. Zero the background using the "Zero" button in the NOMS software.
- Add 10 µl of nitrite standard solution to a 20 ml of calibration solution while stirring. Wait until the current reaches its maximum potential and begins to decline.
- Zero the background again by pressing the "Zero" button in the NOMS software.
- Repeat steps 5-6 at least three more times with adding 20, 40 and 80 µl of nitrite standard solution, respectively.
- Measure the peak height of each addition with the NOMS software by placing the cursor on the peak (panel A, Figure 2). Plot current (pA) vs. concentration of NO/nitrite (nM) to make a reference curve (panel B, Figure 2).
Note: The final volume in the vial is 20 ml, and the NO concentration range is 0-400 nM.
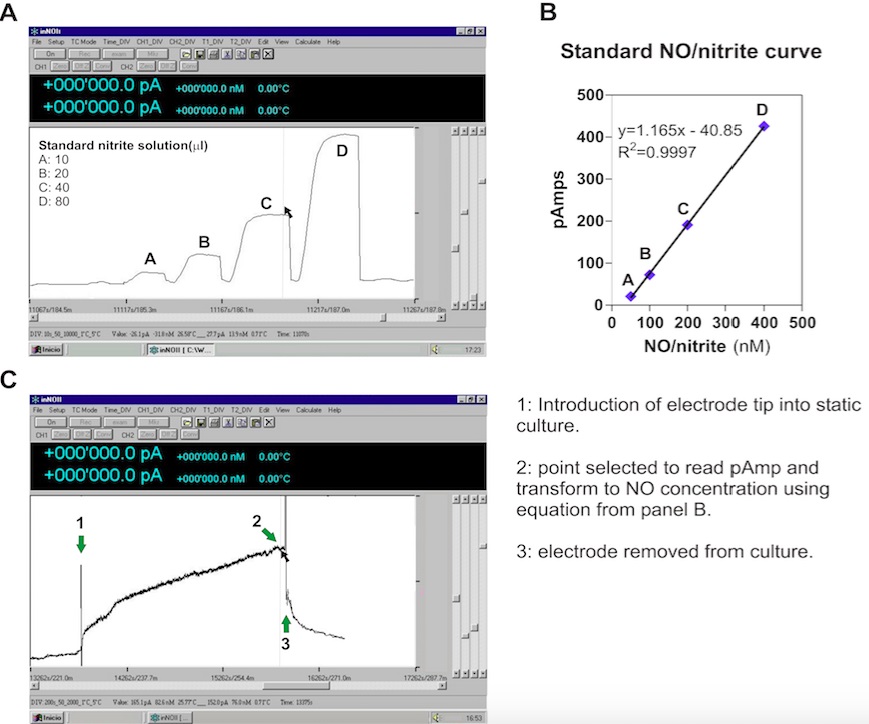
Figure 2. Construction of standard curve (A, B) and determination of NO in A. brasilense Sp245 biofilm sample (C) using a NO electrode
Software
- NOMS software
Procedure
- Grow A. brasilense Sp245 (2 ml per well) on tissue culture plates for 2 d in NFb-malic medium under static conditions to allow biofilm formation.
- Immediately before use, stabilize the microelectrode for 15 min running in Buffer B followed by 15 min in NFb-malic medium.
- Zero the background.
- Immerse microelectrode 3-4 mm into the bacterial culture and start recording changes of current potential. Usually, 30-40 min of recording time is needed per sample to measure NO production in Azospirillum static cultures.
- Enter the obtained current value in the standard curve to establish NO concentration of the samples. Use the equation (panel B, Figure 2) to transform the current values to a concentration of NO. The “y” value is the pA detected for the sample and the “x” value corresponds to NO concentration in nM.
Note: The concentration of nitrite in a sample can be measured in vitro by injecting bacterial culture supernatant into an acid/iodide solution in which nitrite is converted to NO and then detected by the sensor.
Recipes
- Calibration solution
Weigh and dissolve 20 mg of potassium iodide in 15 ml of Milli Q water and 2 ml of 1 M sulfuric acid. After potassium iodine has dissolved completely, add Milli Q water to make 20 ml of solution.
Note: Once this solution becomes light yellow, due to the formation of iodine in the solution, discard and prepare a new solution. - Buffer B
PBS was prepared according to product specifications. One tablet of PBS was dissolved in 200 ml of deionized water to yield 0.01 M phosphate buffer, 0.0027 M potassium chloride and 0.137 M sodium chloride (pH 7.4), at 25 °C. Store at room temperature. - NFb-malic medium [modified as in Arruebarrena et al. (2013)]
For 1 L NFb-malic medium pH 6.5 with nitrate as N source: 3.7 g Malic acid, 5 ml K2HPO4 10% (w/v), 2 ml MgSO4.7H2O 10% (w/v), 1 ml NaCl 10% (w/v), 2 ml CaCl2.H2O 1% (w/v), 2 ml Micronutrients solution, 4 ml Fe-EDTA 1.64% (w/v), 4.5 g KOH, 1.39 g KNO3.
For 200 ml of micronutrients solution: 200 mg NaMoO4.2H2O, 235 mg MnSO4, 280 mg H3BO3, 8 mg CuSO4.5H2O, 24 mg ZnSO4.7H2O.
Method 2. NO Fluorometric assay
Materials and Reagents
- Square Petri dishes (Deltalab, catalog number: 200204 )
- Multi-well plates (12 or 24 wells) (Biofil®)
- Microscopic glass slides (Deltalab, catalog number: D100001 ) and cover slips (Deltalab, catalog number: D102440 )
- Five-day-old Arabidopsis root seedlings, ecotype Columbia Col-0
- Murashige and Skoog Basal Salt Mixture (MS) (Sigma-Aldrich, catalog number: M5524 )
- 1 mM Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: 449709 )
Note: Store at room temperature. - 0.25 mM KCl (Sigma-Aldrich, catalog number: P3911 )
Note: Store at room temperature. - DAF-FM diacetate (DAF-FM DA) (Thermo Fisher Scientific, Molecular probesTM, catalog number: D-23844 )
Note: Store at -20 ºC. - Abscisic acid (Sigma-Aldrich, catalog number: A1049 )
Note: Stoge at -20 ºC. - Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418 )
- 5 mM MES buffer (pH 5.7) (Sigma-Aldrich, catalog number: M3671 )
- DAF-FM diacetate (DAF-FM DA) stock solution (see Recipes)
- Buffer C (see Recipes)
Equipment
- Bright field and fluorescent microscope Eclipse E200 microscope (Nikon Corporation) (http://www.nikon.com/)
Procedure
- Grow Arabidopsis in Petri dishes in ½ strength MS medium for 4 to 5 d. Use tweezers to transfer to microplate wells containing abscisic acid (ABA) 10 µM in ½ strength liquid MS medium for 2 h.
- Replace the ABA solution with 1 ml Buffer C containing 10 µM DAF FM DA (step 1, Figure 3).
- Incubate seedlings at room temperature protected from light for 20 min followed by a wash with 1 ml of fresh Buffer C for 20 min.
- Mount seedlings on glass slides and cover with cover slips. Samples are visualized under bright field and epifluorescent microscopy (excitation 490 nm; emission 525 nm) (step 2, Figure 3).

Figure 3. Nitric Oxide detection by DAF-FA DA in Arabidopsis seedling root
Recipes
- DAF-FM diacetate (DAF-FM DA) stock solution
5 mM DAF-FM DA in dimethyl sulfoxide (DMSO) - Buffer C
Store at room temperature
5 mM MES buffer, adjusted with KOH to pH 5.7
1 mM CaCl2
0.25 mM KCl
Acknowledgments
This research was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT: PICTs 2383/2011 and 2621/2011 to L.L. and N.C.-A., respectively), the Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 0903/2011 to L. L.), and institutional grants from the Universidad Nacional de Mar del Plata, Argentina. This protocol was adapted from the published work (Palma et al., 2013; Foresi et al., 2015).
References
- Allen, B. W. and Piantadosi, C. A. (2003). Electrochemical activation of electrodes for amperometric detection of nitric oxide. Nitric Oxide 8(4): 243-252.
- Arruebarrena Di Palma, A., Pereyra, C. M., Moreno Ramirez, L., Xiqui Vazquez, M. L., Baca, B. E., Pereyra, M. A., Lamattina, L. and Creus, C. M. (2013). Denitrification-derived nitric oxide modulates biofilm formation in Azospirillum brasilense. FEMS Microbiol Lett 338(1): 77-85.
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Coneski, P. N. and Schoenfisch, M. H. (2012). Nitric oxide release: part III. Measurement and reporting. Chem Soc Rev 41(10): 3753-3758.
- Foresi, N., Mayta, M. L., Lodeyro, A. F., Scuffi, D., Correa-Aragunde, N., Garcia-Mata, C., Casalongue, C., Carrillo, N. and Lamattina, L. (2015). Expression of the tetrahydrofolate-dependent nitric oxide synthase from the green alga Ostreococcus tauri increases tolerance to abiotic stresses and influences stomatal development in Arabidopsis. Plant J 82(5): 806-821.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Foresi, N., Correa-Aragunde, N., Amenta, M., Arruebarrena, A., Creus, C. and Lamattina, L. (2016). Detection of Nitric Oxide and Determination of Nitrite Concentrations in Arabidopsis thaliana and Azospirilum brasilense. Bio-protocol 6(6): e1765. DOI: 10.21769/BioProtoc.1765.
Category
Plant Science > Plant physiology > Nutrition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link














