- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Extraction and Measurement of Strigolactones in Sorghum Roots
Published: Vol 6, Iss 6, Mar 20, 2016 DOI: 10.21769/BioProtoc.1763 Views: 11218
Reviewed by: Arsalan DaudiLaia ArmengotAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measurements of Root Colonized Bacteria Species
Vílchez Juan Ignacio [...] Huiming Zhang
Apr 5, 2021 6328 Views

Extraction and Quantification of Plant Hormones and RNA from Pea Axillary Buds
Da Cao [...] Christine A. Beveridge
Oct 5, 2022 2968 Views

Analysis of Modified Plant Metabolites Using Widely Targeted Metabolite Modificomics
Jianing Zhang [...] Jun Yang
Apr 5, 2025 1469 Views
Abstract
Strigolactones (SLs) are carotenoid-derived signaling chemicals containing two lactone moieties in their structures and induce seed germination of root parasitic plants, Striga and Orobanche spp. In the rhizosphere, SLs are essential host recognition signals not only for root parasitic plants but also for arbuscular mycorrhizal fungi. In plants, SLs play important roles as plant hormones regulating shoot and root architecture. Plants produce only trace amounts of chemically unstable SLs, which makes it difficult to determine SL contents in plant tissues. Here, we describe how to extract and quantify sorgomol and 5-deoxystrigol, major SLs produced in sorghum roots.
Keywords: StrigolactoneMaterials and Reagents
- 50 ml Screw cap bottles (MonotaRO Co., Duran, catalog number: 371-05-20-52 )
- Filter paper (90 mm) (MISUMI Corporation, Toyo Roshi Kaisha Ltd, catalog number: 00011090 )
- pH indicator paper (Merck Millipore Corporation, catalog number: 109526 )
- Spin column (Merck Millipore Corporation, catalog number: UFC3 0HV 000 )
- Vials and caps (Chromacol, catalog number: 1030-41201 and 1030-42473 ) for LC-MS/MS analysis
- Sorghum (Sorghum bicolor) plants grown under P or N deficiency
Note: Striga-tolerant sorghum cultivars may be obtained from International Crops Research Institute for the Semi-Arid Tropics (ICRISAT). We examined several sorghum “Hybrid” cultivars for their SL production, and there were essentially no quantitative and qualitative differences in SL production among them. - Ethyl acetate (KANTO CHEMICAL, catalog number: 14029-70 )
- 500 pg of d6-5-deoxystrigol (Ueno et al., 2010) added to each sample in our experiments
Note: Internal standards if available. - Anhydrous MgSO4 (KANTO CHEMICAL, catalog number: 25035-00 ) or Na2SO4 (Kanto Chemical, catalog number: 37280-00 )
- Acetonitrile (KANTO CHEMICAL, catalog number: 01031-2B )
- 0.2 M K2HPO4 (see Recipes)
- Acetonitrile with 0.1% acetic acid for LC-MS/MS (see Recipes)
- Water with 0.1% acetic acid for LC-MS/MS (see Recipes)
Equipment
- Funnel (45 mm diameter) (MonotaRO Co., AGJ, catalog number: 233-09-11-04 )
- Erlenmeyer flasks (50 ml) (Sansyo, Iwaki, catalog number: 4980FK50 )
- Separatory funnel (100 ml) (Iwaki, catalog number: 6402FS100R )
- Evaporator flasks (300 ml, 50 ml) (Sibata, catalog number: 371-13-68-34 and 371-13-68-21 )
- Pasteur pipette (Thermo Fisher Scientific, catalog number: 13-678-20C )
- Mini-benchtop centrifuge (IKA, catalog number: 969-65-03-01 )
- LC-MS/MS (AB Sciex, model: QTRAP 5500 )
- UHPLC system (Shimadzu Corporation, model: Nexera X2 )
- 18 column (ϕ 2.1 x 150 mm, 2.6 μm) (Phenomenex, model: Kinetex C18 )
Procedure
- Add 10-20 ml of ethyl acetate to 50 ml screw cap bottles and measure the weights of the bottles. Ethyl acetate volumes are adjusted to plant volumes so that all plant tissues are covered with ethyl acetate.
- Harvest healthy root tissues (ca. 1 g FW) from 2-4 week-old sorghum plants and immediately put them into the bottles containing ethyl acetate. It is better not to store root tissues in a freezer to avoid possible degradation of SLs. In the case of sorghum, SL production is promoted by N or P deficiency, and thus plants grown under nutrient-deficient conditions contain larger amounts of SLs. It is better to grow plants hydroponically because root tissues are easily harvested without loss. Sorghum plants grow well in hydroponic culture.
- Measure the weight of the bottles again to estimate the weights of collected root tissues for SL extraction.
- Add internal standards if available. We use d6-5-deoxystrigol as an internal standard. Amount of the internal standard should be 1/10 to 10-fold that of endogenous 5-deoxystrigol and therefore it is better to conduct a preliminary experiment to estimate levels of endogenous SL. In general 500 pg is added for 1 g FW root sample. The internal standard is dissolved in acetonitrile or ethyl acetate and added to the bottles containing ethyl acetate.
- Cut root tissues into small pieces (ca. 2-3 mm long) by scissors in ethyl acetate. Acetone can be used for extraction, but acetone extracts need purification before LC-MS/MS analysis.
- Keep the bottles at 4 °C for at least 2 days. However, prolonged storage longer than a week may cause a gradual degradation of SLs.
- Filtrate the solution with the roots with a funnel containing the filter paper. Then, the solution is transferred into the separatory funnel. Add 10 ml of 0.2 M K2HPO4 and mix well. Check pH of aqueous (lower) phase with pH indicator paper. If aqueous phase is still acidic, repeat washing the ethyl acetate solution with 10 ml of 0.2 M K2HPO4.
- Collect ethyl acetate solution in an Erlenmeyer flask and add 1-2 g of anhydrous MgSO4 or Na2SO4.
- Transfer ethyl acetate solution by using funnel and filter paper into an evaporator flask (300 ml) and concentrate in vacuo on a rotary evaporator at below 35 °C.
- Dissolve the residue in a small volume of ethyl acetate (ca. 20 ml in total), transfer to a smaller evaporator flask (50 ml) and concentrate in vacuo.
- Dissolve the residue with 100 µl acetonitrile and transfer the sample solution to a spin column. After centrifugation at 3,000 rpm for 30 sec, transfer filtrate into vials for LC-MS/MS. These samples should be kept at or below 4 °C until use.
- Perform LC-MS/MS analysis (Figure 1).
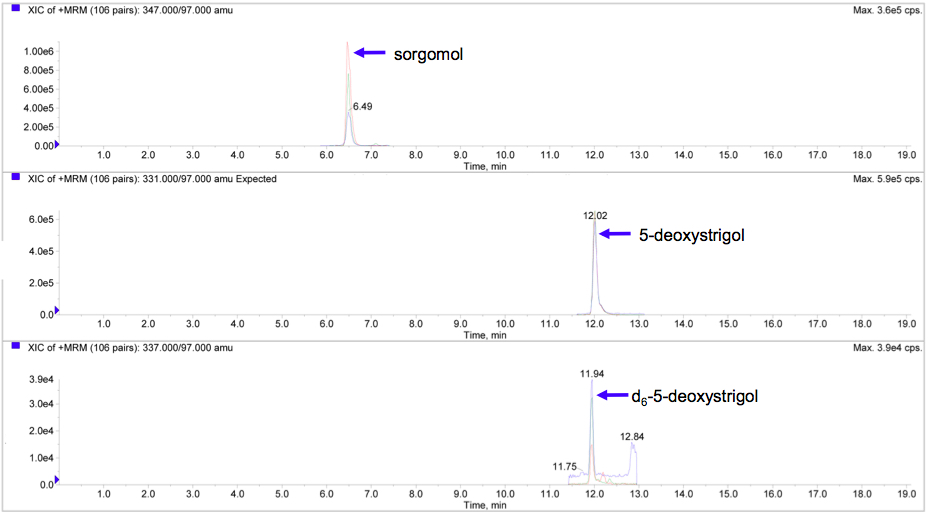
Figure 1. LC-MS/MS analysis of strigolactones in sorghum roots. Product ion scan chromatogram for sorgomol (top), 5-deoxystrigol (middle), and d6-5-deoxystrigol (bottom). - LC-MS/MS analytical conditions for detection and quantification of strigolactones. Analyses of strigolactones were performed using a triple quadrupole/linear ion trap instrument (LIT) with an electrospray ionization (ESI) source and coupled to a UHPLC system.
- Chromatographic separation was achieved on a C18 column (ϕ 2.1 x 150 mm, 2.6 μm;) by applying acetonitrile (MeCN)-H2O contained 0.1% (v/v) acetic acid gradient to the column, starting from 35% MeCN and rising to 95% MeCN at 20.0 min. Finally, the column was equilibrated for 3 min, using this solvent composition. The column was operated at 30 °C with a flow-rate of 0.2 ml/min.
- MS/MS spectra were recorded in product ion scan mode using LIT. Ion source was maintained at 400 °C with curtain gas at 20 psi, collisional activated dissociation (CAD) gas at 7 psi (12 psi for LIT), ion source gas at 80 psi, and ion source gas2 at 70 psi. Ionspray voltage was set at 5,500 V in positive ion mode and -4,500 V in negative ion mode. Declustering, entrance, and collision cell exit potentials were maintained at 60, 10, and 15 V, respectively. One-fifth of the ethyl acetate extract samples dissolved in 2 μl MeCN were injected to the LC-MS/MS.
- The transitions of m/z 347-231, 347-233, and 347-97 were monitored for sorgomol (retention time 6.49 min); m/z 331-234, 331-216, and 331-97 for 5-deoxystrigol (retention time 12.02 min); m/z 337-240, 337-222, and 337-97 for d6-5-deoxystrigol (retention time 11.94 min) in the ESI positive mode (Xie et al., 2015).
Recipes
- 0.2 M K2HPO4
34.8 g K2HPO4
Add dH2O to 1,000 ml
Stored at RT - Acetonitrile with 0.1% acetic acid for LC-MS/MS
Add 500 µl acetic acid to 500 ml acetonitrile
Sonicate for a few minutes
Stored at RT - Water with 0.1% acetic acid for LC-MS/MS
Add 500 µl acetic acid to 500 ml water
Sonicate for a few minutes
Stored at RT
Notes
The root content of SLs varies with nitrogen (N) and phosphorus (P) status of sorghum plant. Under N or P deficiency, root contents of sorgomol and 5-deoxystrigol would be approx. 200 and 300 pg/g root FW, respectively. These values may decrease to 1/100 when the plants are subjected to sufficient N and P. Since sorghum produces relatively large amounts of SLs, single plant (seedling) is enough for SL quantification when grown under N or P deficiency. To minimize an individual difference, it is better to perform experiments with 5 to 10 plants in triplicate.
Acknowledgments
This protocol was adapted from previously published studies, Yoneyama et al. (2013), Yoneyama et al. (2015) and Xie et al. (2015). Kaori Yoneyama was supported by a JSPS Fellowship for young scientists and JSPS Restart Postodoctoral Fellowship.
References
- Ueno, K., Hanada, A., Yamaguchi, S. and Asami, T. (2010). Preparation of multideuterated 5-deoxystrigol for use as an internal standard for quantitative LC/MS. J. Label Compd Radiopharm 53(13): 763-766.
- Xie, X., Yoneyama, K., Kisugi, T., Nomura, T., Akiyama, K., Asami, T. and Yoneyama, K. (2015). Strigolactones are transported from roots to shoots, although not through the xylem. J Pestic Sci 40(4): 214-216.
- Xie, X., Yoneyama, K., Kusumoto, D., Yamada, Y., Takeuchi, Y., Sugimoto, Y. and Yoneyama, K. (2008). Sorgomol, germination stimulant for root parasitic plants, produced by Sorghum bicolor. Tetrahedron Lett 49: 2066-2068.
- Yoneyama K., Kisugi T., Xie X., Arakawa R., Ezawa T., Nomura T. and Yoneyama K. (2015). Shoot-derived signals other than auxin are involved in systemic regulation of strigolactone production in roots. Planta 241: 687-698.
- Yoneyama, K., Xie, X., Kisugi, T., Nomura, T. and Yoneyama, K. (2013). Nitrogen and phosphorus fertilization negatively affects strigolactone production and exudation in sorghum. Planta 238(5): 885-894.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Yoneyama, K., Xie, X., Nomura, T. and Yoneyama, K. (2016). Extraction and Measurement of Strigolactones in Sorghum Roots. Bio-protocol 6(6): e1763. DOI: 10.21769/BioProtoc.1763.
Category
Plant Science > Plant biochemistry > Plant hormone
Plant Science > Plant metabolism > Metabolite profiling
Plant Science > Plant physiology > Endosymbiosis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









