- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Assessment of Immunological Synapse Formation by Flow Cytometry
Published: Vol 6, Iss 6, Mar 20, 2016 DOI: 10.21769/BioProtoc.1758 Views: 17282
Reviewed by: Ralph BottcherMartin V KolevAlka Mehra

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Addressing the Role of Conventional CD8αβ+ T Cells and CD4+ T Cells in Intestinal Immunopathology Using a Bone Marrow–Engrafted Model
Amneh Aoudi [...] Saïdi Soudja
Feb 20, 2024 2969 Views

T-Cell-Based Platform for Functional Screening of T-Cell Receptors Identified in Single-Cell RNA Sequencing Data Sets of Tumor-Infiltrating T-Cells
Aaron Rodriguez Ehrenfried [...] Rienk Offringa
Apr 20, 2024 6409 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3551 Views
Abstract
In adaptive immune system, formation of immunological synapse between T cells and antigen presenting cells (dendritic cells, B cells, and macrophages) or target cells (tumor cells and viral-infected cells) is critical for the execution of T cell immune responses via cytokine secretion or direct killing activity. Here, we describe the practical methods that directly measure the number of conjugates as a result of immunological synapse formation between T cells and superantigen-loaded B cells or between cytotoxic T cells and antigen-loaded target cells by dual-color flow cytometry.
Keywords: T cellsMaterials and Reagents
- 15 ml and 50 ml conical tube
- 12 well cell culture plate
- FalconTM Cell strainer (40 μm Nylon) (Corning, catalog number: 352340 )
- 1 ml syringe
- FalconTM 5 ml Polystyrene Round-Bottom tube (Corning, catalog number: 352052 )
- MACS MS column (Miltenyi Biotec, catalog number: 130-042-201 ) and MACS separator (Miltenyi Biotec)
- Jurkat T cell (ATCC, catalog number: TIB-152 )
- Raji B cell (ATCC, catalog number: CCL-86 )
- EL4 cell (ATCC, catalog number: TIB-39 )
- C57BL/6 mice (8-10 weeks)
- OTI mice (8-10 weeks) (THE JACKSON LABORATORY, catalog number: 021773 )
Note: These mice have the transgenic T cell receptor designed to recognize ovalbumin residues 257-264 in the context of H2Kb. - Staphylococcus Enterotoxin E (SEE) (Toxin Technology, catalog number: ET404 )
- Staphylococcus Enterotoxin B (SEB) (Toxin Technology, catalog number: BT202 )
- OVA 257–264 peptide (InvivoGen, catalog number: vac-sin )
- CellTracker Green CMFDA (Life Technologies, Molecular Probes®, catalog number: C7025 )
Note: Currently, it is “Thermo Fisher Scientific, Molecular ProbesTM, catalog number: C7025”. - CellTracker Orange CMRA (Life Technologies, Molecular Probes®, catalog number: C34551 )
Note: Currently, it is “Thermo Fisher Scientific, Molecular ProbesTM, catalog number: C34551”. - RBC lysis buffer (BioLegend, catalog number: 42030 )
- Mouse CD3+ T cell enrichment column kit (R&D Systems, catalog number: MTCC535 )
- Mouse B cell enrichment kit (STEMCELL Technologies, catalog number: 19754A )
- CD8a (Ly-2) MicroBeads (Miltenyi Biotec, catalog number: 130-049-401 )
- Recombinant human IL-2 (rhIL-2) (NIH AIDS Reagent Program, catalog number: 11697 )
- RPMI1640 (Thermo Fisher Scientific, GibcoTM, catalog number: 31800 )
- 10% heat-inactivated fetal bovine serum (FBS) (Siyaku, Wako Pure Chemical Industries, AusGeneX, catalog number: FBSUS500 )
- Penicillin/streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15140 )
- Non-essential amino acids (Thermo Fisher Scientific, GibcoTM, catalog number: 11140 )
- Sodium pyruvate (Thermo Fisher Scientific, GibcoTM, catalog number: 11360 )
- 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M-7522 )
- Complete RPMI1640 medium (see Recipes)
- Mouse T cell medium (see Recipes)
Equipment
- Water Jacketed CO2 incubator (37 °C, 5% CO2) (Thermo Fisher Scientific)
- FACSCanto instrument (BD Bioscience)
Procedure
- Conjugation assay using cell lines
- Incubate Jurkat T cells (2 x 105/sample) with 1 μM of CellTracker Green CMFDA and Raji B cells (2 x 105/sample) with 3 μM CellTracker Orange CMRA in 0.5-1 ml of complete RPMI1640 medium in 15 ml conical tubes for 30 min at 37 °C in a CO2 incubator.
Note: Perform all steps in the dark to prevent bleaching of fluorescence. - For washing, centrifuge the cells at 1,300 rpm for 3 min, discard the supernatant, and resuspend cell pellet using complete RPMI1640 medium. Repeat twice. After washing, resuspend the cells in 200 μl/sample of complete RPMI1640 medium.
- Treat CMRA-labelled Raji B cells in 200 μl/sample of complete RPMI1640 medium with 5 μg/ml SEE for 30 min at 37 °C.
Notes:- SEE and SEB (B.5) act as the superantigens. Prepare SEE and SEB stock solution of 1 mg/ml in sterile distilled water (SDW) and store in -80 °C.
- Control samples of conjugation assay are samples untreated with SEE, SEB (B), and OVA peptide (C).
- SEE and SEB act as a superantigen that cause non-specific activation of T cells resulting in polyclonal T cell activation and massive cytokine release. Although SEE and SEB can bind both TCR and MHC to induce T cell activation, binding sites are different. SEE is used for formation of conjugation between Jurkat T cell-Raji B cell because Jurkat T cells have the human TCR Vβ recognized by SEE, not SEB. SEE can also apply to form mouse T cell-B cell conjugates, but SEB is more widely used in mouse T cell activation.
- SEE and SEB (B.5) act as the superantigens. Prepare SEE and SEB stock solution of 1 mg/ml in sterile distilled water (SDW) and store in -80 °C.
- Mix and incubate equal cell number of CMFDA-T cells (2 x 105/200 μl/sample) and CMRA-B cells (2 x 105/200 μl/sample) in complete RPMI1640 medium in the polystyrene round-bottom tube for 30 min at 37 °C in a CO2 incubator.
- Analyze the T cell-B cell conjugates using dual-color flow cytometry.
- Prepare the samples as the table below.
Unstained Single-Color Dual-Color T cells B cells/EL4 cells CMFDA (green) labeled T cells CMRA (red) labeled B/EL4 cells CMFDA (green) labeled T cells co-incubated CMRA (orange) labeled B/EL4 cells with or without treatment of SEE/SEB/OVA Set aside 2 x 105 of these cells before starting step A1 for step A5b Set aside 2 x 105 of these cells before starting step A1 for step A5b Set aside 2 x 105 of these cells before step A3 for step A5c Set aside 2 x 105 of these cells before step A3 for step A5c These samples will be used for step A5d - Analyze unstained control to set voltage and gates (FSC, SSC, fluorescence signals).
- Analyze single-color stained controls for compensation.
- After compensation, analyze dual-color stained samples.
Notes:- Read the fluorescence of 10,000-20,000 cells in all controls and samples for analysis.
- The percentage of green-orange events indicates the proportion of T cell-B cell conjugates (Figure 1).
- Read the fluorescence of 10,000-20,000 cells in all controls and samples for analysis.
- Prepare the samples as the table below.
- Incubate Jurkat T cells (2 x 105/sample) with 1 μM of CellTracker Green CMFDA and Raji B cells (2 x 105/sample) with 3 μM CellTracker Orange CMRA in 0.5-1 ml of complete RPMI1640 medium in 15 ml conical tubes for 30 min at 37 °C in a CO2 incubator.
- Conjugation assay using mouse T cells and B cells
- Isolate splenocytes from the spleen from a C57BL/6 mouse.
- Cut out the spleen.
- Mash the spleen through the cell strainer into 50 ml conical tube using the plunger end of the 1 ml syringe.
- Wash the cell strainer with 5-6 ml mouse T cell medium and discard the strainer.
- Transfer the suspended cells into a 15 ml conical tube, and spin down cells at 1,800 rpm for 3 min.
- Discard supernatant and resuspend cell pellet in 1 ml RBC (Red blood cell) lysis buffer to remove RBCs. After 1 min, add 5 ml mouse T cell media and discard any dead cell mass.
- Spin down at 1,800 rpm for 3 min, and discard supernatant. Resuspend pellet in T cell enrichment buffer provided by the manufacturer or B cell enrichment buffer (2% FBS in PBS).
- Cut out the spleen.
- Isolate CD3+ T cells from splenocytes using Mouse CD3+ T cell enrichment column kit according to the manufacturer’s protocol. Isolate B cells from mouse splenocytes using Mouse B cell enrichment kit according to the manufacturer’s protocol. After isolation, resuspend cells in mouse T cell medium.
Note: The yield of T cell and B cell enrichment are above 95%. - Incubate CD3+ T cells with 1 μM of CellTracker Green CMFDA (2 x 105/sample) and B cells with 3 μM CellTracker Orange CMRA (2 x 105/sample) in 0.5-1 ml of mouse T cell medium at 37 °C in 15 ml conical tubes as described in step A1.
- For washing, centrifuge the cells at 1,800 rpm for 3 min, discard the supernatant, and resuspend cell pellet using mouse T cell medium. Repeat twice. After washing, resuspend the cells in 200 μl/sample of mouse T cell medium.
- Treat B cells with 5 μg/ml SEB in 200 μl/sample of mouse T cell medium for 30 min at 37 °C.
- Mix and incubate equal cell number in 200 μl/sample of mouse T cell medium of CMFDA-CD3+ T cells and CMRA-B cells in the polystyrene round-bottom tube for 30 min at 37 °C in a CO2 incubator.
- Analyze the T cell-B cell conjugates using dual-color flow cytometry as described in step A5.
- Isolate splenocytes from the spleen from a C57BL/6 mouse.
- Conjugation assay using antigen-specific cytotoxic T cells and target cells
- Isolate splenocytes from an OTI mouse as described in step B1, and resuspend splenocytes in mouse T cell medium.
Note: CD8+ T cells purified from an OTI-transgenic TCR mice express Ova-specific TCR that can recognize OVA 257-264 peptide as an antigen. - For differentiation to OVA-specific cytotoxic T cells, stimulate splenocytes (2 x 106 cells/well) with OVA 257-264 peptide in mouse T cell medium with 10 U/ml rhIL-2 in 12 well cell culture plates for 2-3 days. Add rhIL-2 to media daily.
Note: Thaw frozen stock of rhIL-2 (kept at -80 °C) and dilute in mouse T cell medium. Diluted rhIL-2 can be stored at 4 °C for one month. - Isolate OVA-specific cytotoxic T cells by MACS separation using CD8a (Ly-2) MicroBeads according to the manufacturer’s protocol.
- Incubate OVA-specific cytotoxic T cells with 1 μM of CellTracker Green CMFDA (2 x 105-1 x 106/sample) and EL4 cells (as target cells; 2 x 105/sample) with 3 μM CellTracker Orange CMRA at 37 °C in mouse T cell medium, wash, and resuspend in mouse T cell medium as described in steps B3-4.
Note: The relevant ratio of cytotoxic T cells and EL4 cells is 1:1-5:1. - Add 10 μg/ml OVA 257–264 peptide to EL4 cells in 200 μl/sample of mouse T cell medium for 30 min at 37 °C.
Note: OVA peptide loaded on EL4 cells acts as the antigen recognized by OVA-specific cytotoxic T cells. - Mix and incubate equal cell number in 200 μl/sample of mouse T cell medium of CMFDA-cytotoxic T cells and CMRA-EL4 cells in the polystyrene round-bottom tube for 30 min-1 h at 37 °C in a CO2 incubator.
- Analyze the cytotoxic T cell-target cell conjugates using dual-color flow cytometry as described in step A5.
- Isolate splenocytes from an OTI mouse as described in step B1, and resuspend splenocytes in mouse T cell medium.
Representative data
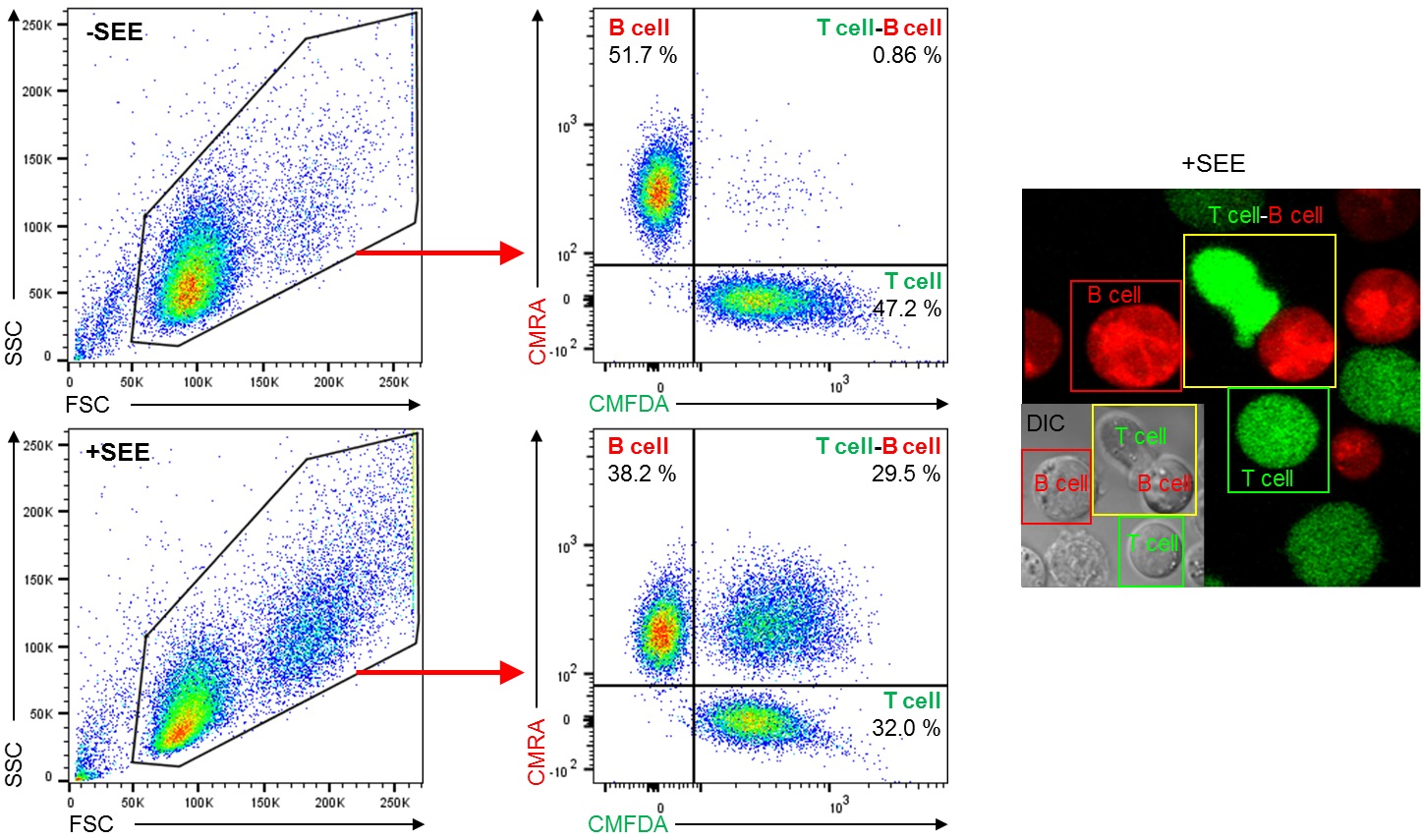
Figure 1. SEE-induced Jurkat T cell-Raji B cell conjugates formation. Conjugates formation using Jurkat T cells and Raji B cells with or without SEE were performed as described in Procedure section A and then analyzed by flow cytometry. Dot plot showing FSC and SSC is gated to exclude cell debris and select cells for dual-color analysis (Left). Cells inside the gate were analyzed by CMFDA (green; T cell) and CMRA (red; B cell) fluorescence signals (middle dot plots). Quadrants are established using negative control and single-color controls. CMFDA+ CMRA- cells (lower right quadrant) indicate T cell only, CMFDA- CMRA+ cells (upper left quadrant) indicate B cell only, and CMFDA+ CMRA+ cells (upper right quadrant) indicate T cell-B cell conjugates. After loading of SEE by Raji B cells (+SEE), T cell-B cell conjugates were increased in compared with SEE-unloaded B cells (-SEE). Numbers in dot plots indicate the percentage of each quadrant. Confocal fluorescence image and differential interference contrast (DIC) image show T cell only (green box), B cell only (red box), and T cell-B cell conjugate (yellow box) after conjugation assay with SEE (Right).
Recipes
- Complete RPMI1640 medium
RPMI1640
10% heat-inactivated fetal bovine serum
100 U/ml Penicillin/streptomycin - Mouse T cell medium
RPMI1640
10% heat-inactivated fetal bovine serum
100 U/ml Penicillin/streptomycin
1% non-essential amino acids
1 mM Sodium pyruvate
50 μM 2-Mercaptoethanol
Acknowledgments
This work was supported by the Basic Science Program (2015R1A2A1A15052658), and the Creative Research Initiative Program (2015R1A3A2066253) through the National Research Foundation (NRF) grants funded by the Ministry of Science, ICT & Future Planning (MSIP), Korea, and Bioimaging Research Center at GIST.
References
- Na, B. R., Kim, H. R., Piragyte, I., Oh, H. M., Kwon, M. S., Akber, U., Lee, H. S., Park, D. S., Song, W. K., Park, Z. Y., Im, S. H., Rho, M. C., Hyun, Y. M., Kim, M. and Jun, C. D. (2015). TAGLN2 regulates T cell activation by stabilizing the actin cytoskeleton at the immunological synapse. J Cell Biol 209(1): 143-162.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Na, B. and Jun, C. (2016). In vitro Assessment of Immunological Synapse Formation by Flow Cytometry. Bio-protocol 6(6): e1758. DOI: 10.21769/BioProtoc.1758.
- Na, B. R., Kim, H. R., Piragyte, I., Oh, H. M., Kwon, M. S., Akber, U., Lee, H. S., Park, D. S., Song, W. K., Park, Z. Y., Im, S. H., Rho, M. C., Hyun, Y. M., Kim, M. and Jun, C. D. (2015). TAGLN2 regulates T cell activation by stabilizing the actin cytoskeleton at the immunological synapse. J Cell Biol 209(1): 143-162.
Category
Immunology > Immune cell staining > Flow cytometry
Immunology > Immune cell function > Lymphocyte
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











