- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Testing the Effect of UV Radiation on the Survival of Burkholderia glumae
Published: Vol 6, Iss 5, Mar 5, 2016 DOI: 10.21769/BioProtoc.1755 Views: 10355
Reviewed by: Zhaohui LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

SIMBA Method—Simultaneous Detection of Antimicrobial and Anti-biofilm Activity of New Compounds Using Salmonella Infantis
Meta Sterniša [...] Anja Klančnik
Aug 5, 2023 2022 Views

Functional Assay for Measuring Bacterial Degradation of Gemcitabine Chemotherapy
Serkan Sayin and Amir Mitchell
Sep 5, 2023 1745 Views

Identification of Mycobacterium tuberculosis and its Drug Resistance by Targeted Nanopore Sequencing Technology
Chen Tang [...] Guangxin Xiang
Feb 5, 2025 1998 Views
Abstract
Burkholderia glumae (B. glumae) is becoming a serious threat in the major rice producing areas of the world. It was reported that Burkholderia spp., including B. glumae, are adapted to a wide range of ecological niches. Different bacterial strains show different levels of UV tolerance which may be due to the presence of different protection mechanisms. Previously we reported that pigment producing strains of B. glumae are more tolerant to UV radiation than non-pigmented strains. Here, we describe the protocol of UV tolerance assay for B. glumae in different exposure times. Using this protocol, we can calculate the survival rate of B. glumae strains, as well as other bacterial species, in exposure to UV radiation.
Materials and Reagents
- Microcentrifuge tubes
- Cuvettes (BrandTech Macro Methacrylate Cuvette) (Cole-Parmer, catalog number: 759081D )
- Spreaders
- Bacterial inoculation loops
- A metal inoculation needle or sterile tooth picks
- Strains of Burkholderia glumae (or other bacterial species)
- Luria-Bertani broth (Sigma-Aldrich, catalog number: L3522 )
- Bacteriological agar (Sigma-Aldrich, catalog number: A5306 )
- Bacto Peptone (BD, DifcoTM, catalog number: 211677 )
- Glucose (Sigma-Aldrich, catalog number: G5400 )
- Casamino acid (Thermo Fisher Scientific, catalog number: BP1424 )
- Nitrofurantoin (MP Biomedicals, catalog number: 155881 )
- Luria-Bertani broth (LB) agar plates (see Recipes)
- Casamino acid-peptone-glucose (CPG) agar plates (see Recipes)
Equipment
- Laminar hood installed with a UV light bulb (The Baker Company, EdgeGard®)
- Spectrophotometer (Thermo Fisher Scientific, model: BioMate3 )
- Incubator (GMI, New Brunswick Scientific, model: Innova 4230 )
- Germicidal fluorescent bulb, 15 W (General Electric Company, model: G15T8 )
Procedure
Note: For handling bacterial cultures, aseptic techniques must be used and each step should be carried out in a sterile laminar flow hood when practicable.
Day 1
- Streak the bacterial cells from glycerol stock on an LB agar plate supplemented with nitrofurantoin (100 µg/ml), an antibiotic to which B. glumae is naturally resistant. 500x stock solution of nitrofurantoin (50 ml/ml) should be prepared by dissolving in dimethylformamide (DMF).
- Incubate the LB plate at 37 °C (optimal temperature for bacterial growth) overnight (~ 18 h).
Day 2
- Streak the overnight-grown bacterial cells from a single colony on a CPG agar plate, using a metal inoculation needle or a sterile toothpick. Incubate the inoculated CPG agar plate at 30 °C (optimal temperature for the production of pigment, which is a main determinant for bacterial tolerance to UV) for 48 h (Figure 1).
Figure 1. Strains of Burkholderia glumae grown on CPG agar plates. The upper-left and bottom plates: Pigment-producing strains of B. glumae. The upper-right plate: A pigment-deficient strain of B. glumae. Photo was taken after 48 h of incubation at 30 °C.
Day 4
- Resuspend the bacterial colonies grown on CPG agar in sterile water in a sterile microcentrifuge tube by flicking with hands.
- Adjust the bacterial concentration to OD600 = 0.1 (~0.5 x 107 CFU/ml), using a spectrophotometer.
- Dilute the bacterial suspension with 1:10 ratio in sterile dH2O.
- Spread 100 µl of the diluted B. glumae cells on a CPG plate.
- Expose B. glumae cells to UV light from a G15T8 germicidal fluorescent bulb, 15 W in a laminar hood at the distance of 70 cm with 253.7 nm wave length for various durations (Figure 2). The lid should be off during the UV exposure.
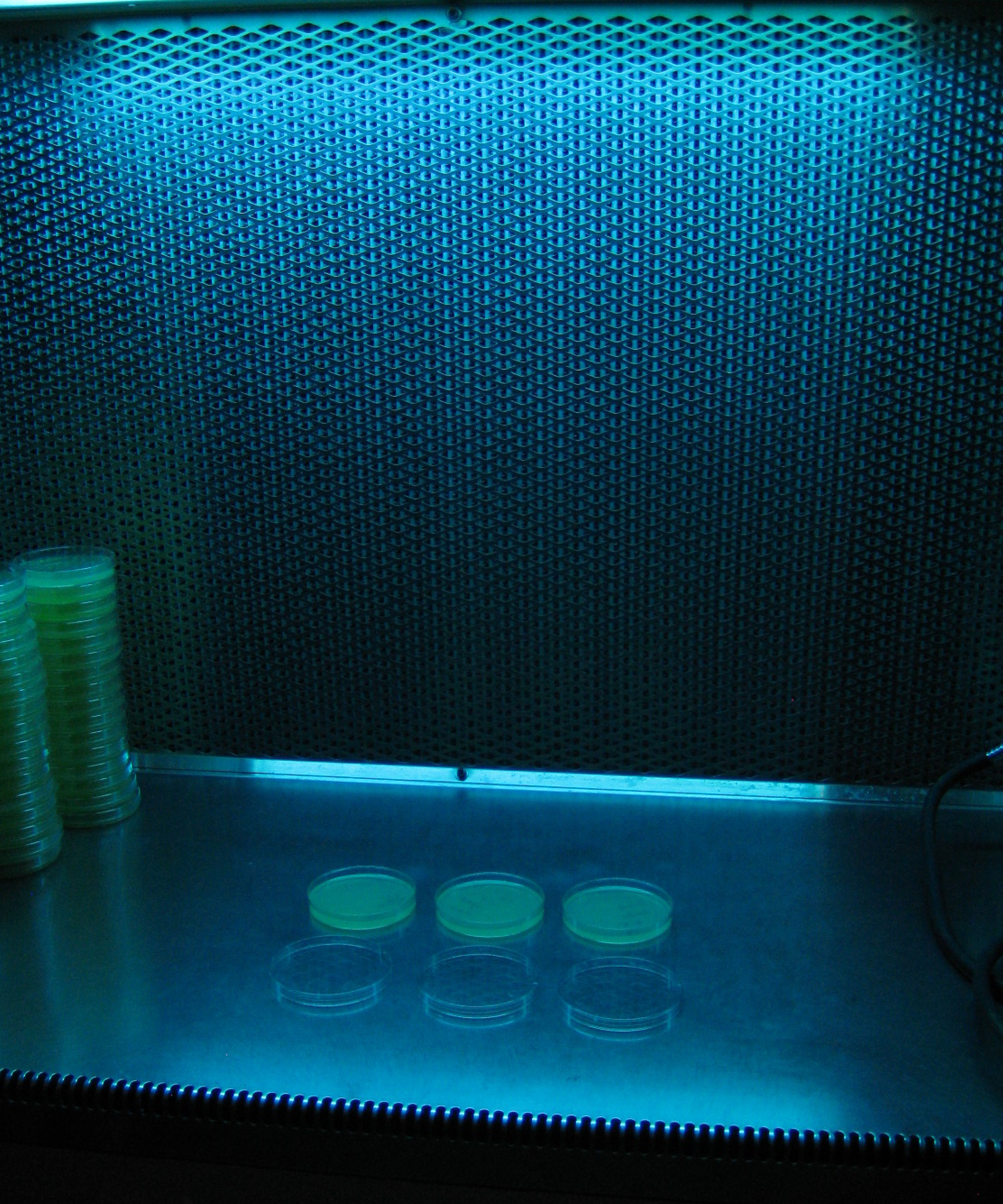
Figure 2. An image showing the UV irradiation procedure - To determine the number of CFU accurately, serial dilutions up to 1:1,000 should be made and spread on CPG agar plates
- Incubate the inoculated plates at 30 °C for 48 h.
Day 6
- Count the surviving bacterial colonies (Figure 3).

Figure 3. An image of bacterial colonies that survived UV exposure. The upper two rows: pigment-producing strains of Burkholderia glumae. The botton row: A pigment-deficient strain of B. glumae. From left to right: Each vertical row represents the CPG agar plates exposed to UV for 0, 10, 20, 40, and 60 sec, respectively. - Convert the data to percentage of survival in different time of exposure to UV radiation.
Survival rate (%) = # colonies from UV-exposed cells/# colonies from non UV-exposed cells x 100
Recipes
- LB Agar plates (1L)
25 g of LB broth
18 g of agar
Add dH2O to 1 L
Sterilize by autoclaving on liquid cycle - CPG agar plates (1 L)
1.5 g of Casamino acid
10 g of Peptone
5 g of Glucose
18 g of agar
Add dH2O to 1 L
Sterilize by autoclaving on liquid cycle
Acknowledgments
This work was supported by the USDA NIFA (Hatch Project #: LAB93918 and LAB94203), the Louisiana State University Agricultural Center, and the Louisiana Board of Regents (the Research and Development Program: LEQSF(2008-11)-RD-A-02). This protocol was modified from our previous work published in PLoS ONE (Karki et al., 2012).
References
- Karki, H. S., Shrestha, B. K., Han, J. W., Groth, D. E., Barphagha, I. K., Rush, M. C., Melanson, R. A., Kim, B. S. and Ham, J. H. (2012). Diversities in virulence, antifungal activity, pigmentation and DNA fingerprint among strains of Burkholderia glumae. PLoS One 7(9): e45376.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Karki, H. S. and Ham, J. H. (2016). Testing the Effect of UV Radiation on the Survival of Burkholderia glumae. Bio-protocol 6(5): e1755. DOI: 10.21769/BioProtoc.1755.
Category
Microbiology > Antimicrobial assay > Antibacterial assay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










