- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Shoot Regenerative Capacity Assays in Arabidopsis and Tobacco
Published: Vol 6, Iss 5, Mar 5, 2016 DOI: 10.21769/BioProtoc.1753 Views: 11091
Reviewed by: Marisa RosaYuan ChenAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Live Imaging of the Shoot Apical Meristem of Intact, Soil-Grown, Flowering Arabidopsis Plants
Gabriele Bradamante
Jun 20, 2024 2286 Views

Micrografting Technique of Hevea brasiliensis In Vitro Plantlets
Florence Dessailly [...] Julie Leclercq
Feb 20, 2025 1491 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1896 Views
Abstract
Plant regeneration refers to a process through which an explant is differentiated to a whole plant under certain conditions. It has been shown that two plant hormones, auxin and cytokinin, play critical roles within this process (Duclercq et al., 2011). Cytokinin induces shoot regeneration, whereas auxin promotes root production. In addition to hormones, recent study has revealed that age cue serves as a common element behind plant cell totipotency (Toledano et al., 2012). Here we present an easy protocol to assess the shoot regenerative capacity of Arabidopsis and tobacco leaves of different ages.
Keywords: Shoot regenerationMaterials and Reagents
- Surgical tape (3M, catalog number: 15300 )
- Sterile petri dishes
- Seeds: Arabidopsis thaliana (Col-0 ecotype) and tobacco (Nicotiana tabacum cv SR1)
- Bacterial strain: Agrobacterium tumefaciens EHA105
- Sodium hypochlorite (Sigma-Aldrich, catalog number: L099100 )
Note: The Pricing and availability are not currently available. - Kanamycin sulfate Streptomyces kanamyceticus (Sigma-Aldrich, catalog number: K0879 )
- Timentin (Yeasen, catalog number: 60230ES07 )
- Agar (Sangon Biotech, catalog number: A100637 )
- Methylester sulfonate (Sangon Biotech, catalog number: MB0341 )
- Sucrose (Sigma-Aldrich, catalog number: E001888 )
- Glucose (Sigma-Aldrich, catalog number: G8270 )
- Ethyl alcohol, Pure (Sigma-Aldrich, catalog number: 459844 )
- Deionized water
- Soil
- Murashige and Skoog (MS) basal medium with vitamin powder (PhytoTechnology, Laboratories®, catalog number: 15B0519117B ) (1 L) (see Recipes)
- 1/2 MS medium (1 L) (see Recipes)
- MS1 medium (1 L) (see Recipes)
- MS2 medium (1 L) (see Recipes)
- MS3 medium (1 L) (see Recipes)
- Callus-inducing medium (1 L) (see Recipes)
- Shoot-inducing medium (1 L) (see Recipes)
- 22 mM 2, 4-dichlorophenoxy (2, 4-D) stock solution (Sigma-Aldrich, catalog number: 31518 ) (see Recipes)
- 9 mM 3-indoleacetic acid (IAA) (Sigma-Aldrich, catalog number: I2886 ) stock solution (see Recipes)
- 20 mM Kinetin (Sigma-Aldrich, catalog number: K0753 ) stock solution (see Recipes)
- 50 mM 6-(γ, γ-Dimethylallylamino)purine (2-IP) (Sigma-Aldrich, catalog number: D5912 ) stock solution (see Recipes)
- 2 mg/ml 6-benzylaminopurine (6-BA) (Sigma-Aldrich, catalog number: B3408 ) stock solution (see Recipes)
- 1 M Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: P5958 ) (see Recipes)
- 15% bleach (see Recipes)
- 10% bleach (see Recipes)
- Infection buffer (see Recipes)
Equipment
- Clean bench (horizontal laminar flow)
- Autoclave (ZEALWAY)
- Incubator (25 °C/80 UML) (Percival-scientific, model: CU-41L4 )
- Growth chamber
- Glass culture bottles (130 mm x 100 mm x 62 mm)
- Sterile 5 mm-hole punch
- Scalpel (10 cm in length)
- Forceps (20 cm in length)
- Scissor (15 cm in length)
Procedure
- Generation of transgenic tobacco plants
To study the molecular basis which age regulates plant regeneration capacity, transgenic tobacco plants with gain of function or loss of function candidate genes were generated by using this method (such as miR156 and its targeted gene SPL9).- To get aseptic seedlings, tobacco seeds were sterilized with 15% bleach to shake for 15 min in 2.0 ml tubes. Then wash three times with sterile water. Every seed was germinated on each ½ MS glass bottle. The numbers of aseptic seedlings were determined by the numbers of transgenes. About five aseptic seedlings were needed in each transgene. Seal the glass bottles using surgical tape. Do these in a clean bench. The glass bottles were incubated for 4-5 weeks at 25 °C under long-day conditions (16 h light/8 h dark) (Figure 1A).
- Cut the aseptic leaves into pieces (about 1 cm in diameter) using scalpel and forceps. Transfer leaf pieces to MS plates and pre-culture them for 2 days at 25 °C under long-day conditions. Do these in a clean bench.
- The candidate genes were cloned into binary vector (such as pCAMBIA2301) and then were delivered into Agrobacterium tumefaciens EHA105 by the freeze-thaw method (Weigel and Glazebrook, 2006) and cultures were incubated for 3 days at 28 °C.
- The single positive colony was incubated into 6 ml liquid LB with relevant antibiotics and shook overnight at 28 °C.
- Transfer 2 ml cultured Agrobacterium to 100 ml fresh LB with relevant antibiotics and grown with shaking overnight at 28 °C.
- The overnight culture of Agrobacterium was centrifuged for 15 min at 4,000 rpm and resuspended in infection buffer (30 g/L glucose, OD600 = 0.8-1.0).
- The pre-cultured leaf pieces were transferred into Agrobacterium suspension and infected for 20~30 min. After that, the infected leaf pieces were transferred to MS1 plates and co-cultured for 2 days at 25 °C under long-day conditions. Do these in a clean bench.
- The infected leaf pieces were transferred to MS2 plate which contained 6-benzylaminopurine (6-BA), a synthetic cytokinin, to induce the formation of shoots. Then explants were cultured for 3-4 weeks at 25 °C under long-day conditions.
- The regenerated adventitious buds (about 1 cm size) on each leaf pieces were isolated by scalpel and forceps (Figure 1B), transferred to a glass culture bottle with MS3 media and cultured for 3-4 weeks.
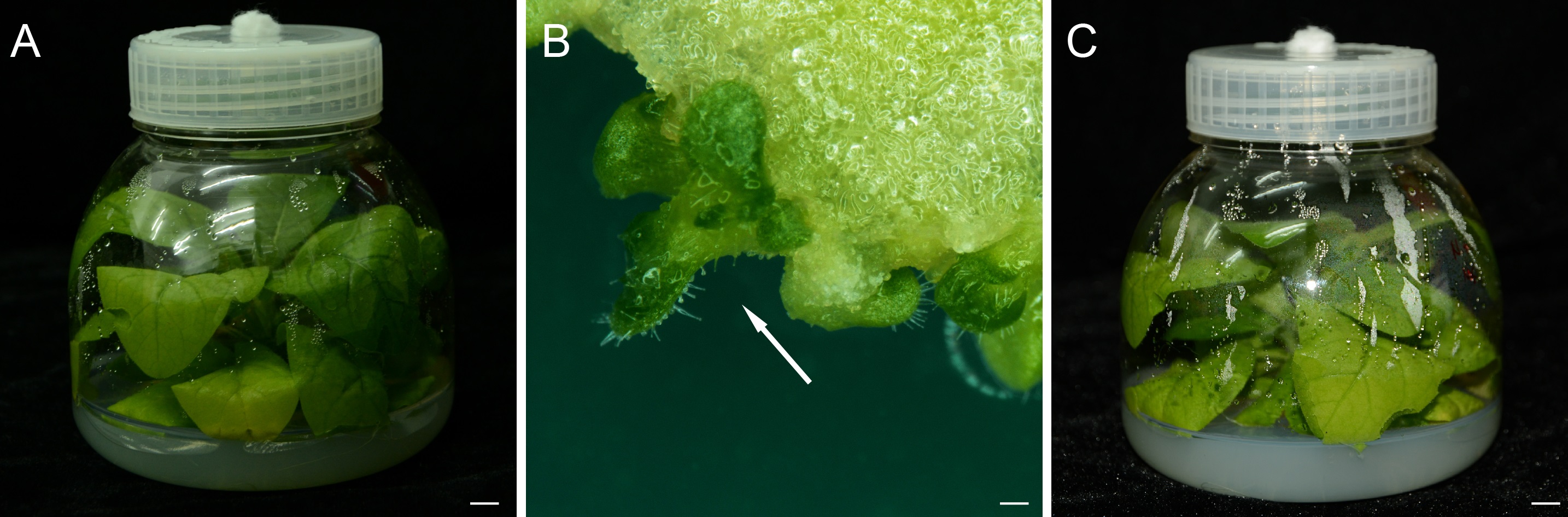
Figure 1. Construct the transgenic plants in tobacco by the tissues cultures. A. A tobacco plant grown in glass bottle for 4-5 weeks, bar = 1 cm. B. The regenerated adventitious buds on an explant, bar = 100 μm. C. A regenerated plant which has been cultured 3-4 weeks on MS3 plate, bar = 1 cm. - Subculture once with MS3 media, when the adventitious buds grew up into whole plants.
- Finally, transfer regenerated plants from MS3 plates to soil and grow them in growth chamber at 25 °C under long-day conditions.
- To get aseptic seedlings, tobacco seeds were sterilized with 15% bleach to shake for 15 min in 2.0 ml tubes. Then wash three times with sterile water. Every seed was germinated on each ½ MS glass bottle. The numbers of aseptic seedlings were determined by the numbers of transgenes. About five aseptic seedlings were needed in each transgene. Seal the glass bottles using surgical tape. Do these in a clean bench. The glass bottles were incubated for 4-5 weeks at 25 °C under long-day conditions (16 h light/8 h dark) (Figure 1A).
- Regeneration assays using the leaves from tobacco plants of different ages
To study the relationship between the shoot regenerative capacity and plant age, regeneration assays were performed using the first/second (early), fifth (mid), and ninth/tenth (late) tobacco leaves as explants.- Tobacco seeds were grow on soil in a growth chamber under long-day conditions.
- The first/second (early), fifth (mid), and ninth/tenth (late) tobacco leaves were collected and placed on deionized water. To avoid the impact of leaf development on regenerative capacity, the leaves of the same size (1 cm in length) were used.
- Detached leaves were sterilized with 10% bleach for 15 min in sterile Petri dishes. Wash three times with sterile water.
- Punch on leaves. The leaf dices without vascular tissue (5-mm in diameter) were used as explant.
- Transfer explants to MS media with 6-BA of different concentrations (such as: 0.05 mg/L, 0.2 mg/L, and 0.5 mg/L). Explants were cultured at 25 °C in an incubator under long-day conditions.
- After about 3-4 weeks, the regenerated shoots appeared. The numbers of explants and regenerated shoots were scored (Figure 2). The regenerative capacity was represented by the number of regenerated shoots in the number of explants.
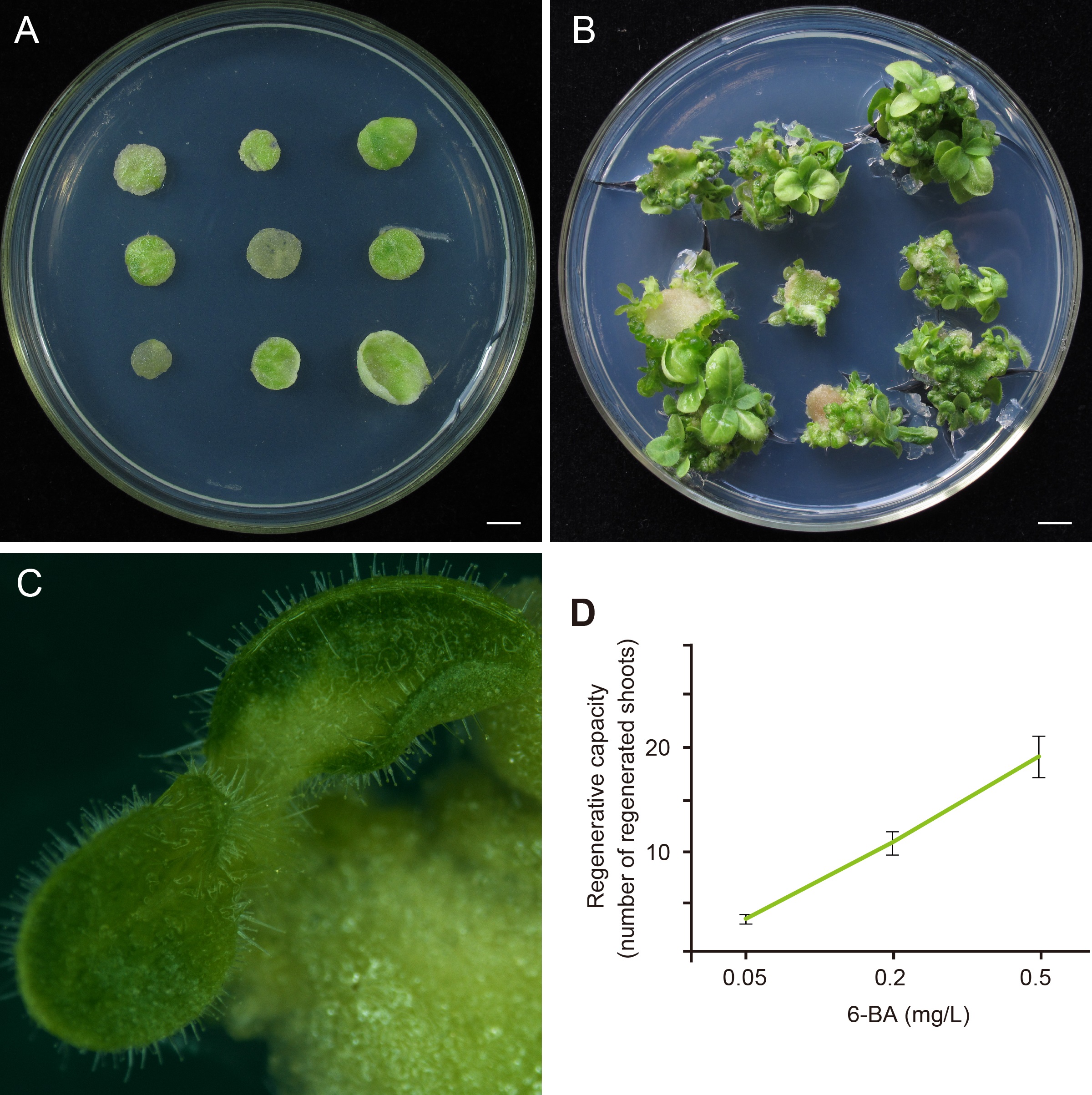
Figure 2. Count the regenerated shoots and evaluate the shoot regeneration capacity of explants in tobacco. A. Explants were cultured on MS plates for 3-4 weeks, n = 3 x 9, bar = 0.5 cm. B. The regenerated shoots from explants which were cultured on the MS media with 6-BA, n = 3 x 9, bar = 0.5 cm. C. Shoots that was scored like this, bar = 100 μm. D. One example of representative data on tobacco.
- Tobacco seeds were grow on soil in a growth chamber under long-day conditions.
- Arabidopsis shoot regeneration assays
In Arabidopsis, shoot regeneration requires two steps. First, explants were cultured on an auxin-rich callus-inducing medium so that they acquired competence to form shoots. Second, the callus were induced to produce shoots on a shoot-induction medium (Valvekens et al., 1988).- Arabidopsis seeds were sterilized with 15% bleach for 15 min and kept at 4 °C for 2 days under darkness.
- The seeds were sowed on 1/2 MS plate and incubated at 22 °C for 7 days under darkness.
- The hypocotyls (1 cm in length) were cut out and used as explants for regeneration assays.
- The hypocotyl segments were transferred to auxin-rich callus-inducing medium (CIM) and cultured for 7 days 22 °C in an incubator under long-day conditions (16 h light/8 h dark) (Figure 3A).
- The calli were then transferred to shoot-inducing medium (SIM) with 2-IP of different concentrations. The shoots will be regenerated by culturing calli at 22 °C under long-day conditions (Figure 3B).
- The number of regenerated shoots was scored. The regenerative capacity was represented by the number of regenerated shoots in number of explants.
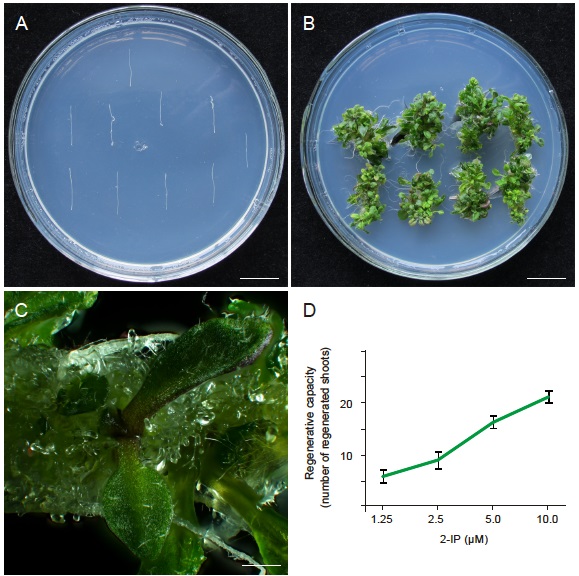
Figure 3. The regeneration assays to measure the shoot regeneration capacity of explants in Arabidopsis. A. Arabidopsis hypocotyls were cultured on an auxin-rich callus-inducing medium for 7 days, n = 3 x 10, bar = 1.0 cm. B. The regenerated shoots on shoot-induction medium, n = 3 x 8, bar = 1.0 cm. C. Shoots that was scored like this, bar = 1.0 cm. D. One example of representative data on Arabidopsis.
- Arabidopsis seeds were sterilized with 15% bleach for 15 min and kept at 4 °C for 2 days under darkness.
Recipes
- MS medium (1 L)
4.46 g of MS basal medium with vitamin powder
0.5 g methylester sulfonate
20 g sucrose
8 g agar
Adjust pH 5.7 with 1 M KOH and autoclave at 121 °C for 15 min - 1/2 MS medium (1 L)
2.23 g of MS basal medium with vitamin powder
0.5 g methylester sulfonate
8 g agar
Adjust pH 5.7 with 1 M KOH and autoclave at 121 °C for 15 min - MS1 medium (1 L)
MS medium with 6-BA 2 mg/L - MS2 medium (1 L)
MS medium with 6-BA 2 mg/L
100 mg/L kanamycin
250 mg/L timentin - MS3 medium (1 L)
MS medium with 100 mg/L kanamycin
250 mg/L timentin - Callus-inducing medium (1 L)
MS medium with 2.2 µM 2,4-D and 0.2 µM kinetin - Shoot-inducing medium (1 L)
MS medium with 0.9 µM IAA and different concentrations of 2-IP - 22 mM 2,4-D stock solution
Dissolve 0.049 g 2,4-D powder in 10 ml ethanol
Filter sterilization and stored at -20 °C - 9 mM IAA stock solution
Dissolve 0.016 g IAA powder in 10 ml ethanol
Filter sterilization and stored at -20 °C - 20 mM kinetin stock solution
Add 0.043 g kinetin powder to <10 ml deionized water and dissolve completely by 1 M
KOH
Adjust volume to 10 ml
Filter sterilization and stored at -20 °C - 50 mM 2-IP stock solution
Add 0.10 g 2-IP powder to <10 ml deionized water and dissolve completely by 1 M KOH
Adjust volume to 10 ml
Filter sterilization and stored at -20 °C - 2 mg/ml 6-BA stock solution
Dissolve 20 mg 6-BA powder in 10 ml DMSO
Filter sterilization and stored at -20 °C - 1 M KOH
Dissolve 5.61 g KOH powder in 100 ml deionized water
Stored at room temperature - 15% bleach
Add 7.5 ml sodium hypochlorite solution into 42.5 ml deionized water - 10% bleach
Add 5 ml sodium hypochlorite solution into 45 ml deionized water - Infection buffer
Dissolve 30 g glucose powder in 1 L deionized water
Acknowledgments
The work in the Wang laboratory is supported by grants from the National Natural Science Foundation of China (31430013; 31222029; 912173023), the State Key Basic Research Program of China (2013CB127000), the Shanghai Outstanding Academic Leader Program (15XD1504100) and the NKLPMG Key Research Program. This protocol was adapted from our recent publications (Zhang et al., 2015a; Zhang et al., 2015b).
References
- Duclercq, J., Sangwan-Norreel, B., Catterou, M. and Sangwan, R. S. (2011). De novo shoot organogenesis: from art to science. Trends Plant Sci 16(11): 597-606.
- Toledano, H., D'Alterio, C., Czech, B., Levine, E. and Jones, D. L. (2012). The let-7-Imp axis regulates ageing of the Drosophila testis stem-cell niche. Nature 485(7400): 605-610.
- Valvekens, D., Van Montagu, M. and Van Lijsebettens, M. (1988). Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci U S A 85(15): 5536-5540.
- Weigel, D. and Glazebrook, J. (2006). Transformation of agrobacterium using the freeze-thaw method. CSH Protoc 2006(7).
- Zhang, T., Wang, J. and Zhou, C. (2015a). The role of miR156 in developmental transitions in Nicotiana tabacum. Sci China Life Sci 58(3): 253-260.
- Zhang, T. Q., Lian, H., Tang, H., Dolezal, K., Zhou, C. M., Yu, S., Chen, J. H., Chen, Q., Liu, H., Ljung, K. and Wang, J. W. (2015b). An intrinsic microRNA timer regulates progressive decline in shoot regenerative capacity in plants. Plant Cell 27(2): 349-360.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, T. and Wang, J. (2016). Shoot Regenerative Capacity Assays in Arabidopsis and Tobacco. Bio-protocol 6(5): e1753. DOI: 10.21769/BioProtoc.1753.
Category
Plant Science > Plant developmental biology > General
Plant Science > Plant physiology > Plant growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link