- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Semi-thin Sectioning, Light and Fluorescence Microscopy of Floral Bud to Study Microspore Development in Arabidopsis
Published: Vol 6, Iss 5, Mar 5, 2016 DOI: 10.21769/BioProtoc.1752 Views: 10180
Reviewed by: Samik BhattacharyaVinay PanwarAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Modified Pseudo-Schiff Propidium Iodide for Staining the Shoot Apical Meristem in Arabidopsis
Ruiqi Li [...] Ligeng Ma
May 5, 2023 2273 Views

A Novel Imaging Protocol for Investigating Arabidopsis thaliana Siliques and Seeds Using X-rays
Brylie A. Ritchie [...] Ansul Lokdarshi
Oct 5, 2023 2189 Views

Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.)
Pradeep Chand Deo
Oct 20, 2025 1438 Views
Abstract
Pollen grains are male gametophytes produced within the pollen sacs of the anthers of the flower. Recent genetic studies have revealed several components involved in microspore development (Borg et al., 2009; Berger and Twell, 2011), and yet many components controlling microspore development remain to be identified. Semi-thin sectioning of anthers and light and fluorescence microscopy of floral bud (Kim et al., 2015) are the initial key experiments to characterize Arabidopsis mutants and transgenic plants for understanding the roles of new genetic components during microspore development. Herein, we describe a protocol for semi-thin sectioning of anthers and light and fluorescence microscopy of floral bud in Arabidopsis.
Keywords: PollenMaterials and Reagents
- 0.2 μm sterile syringe filter (Corning, catalog number: 431219 )
- Microscope slide and coverslip
- Needle (30 gauge)
- 3 mm Whatman filter paper (GE Healthcare, Dharmacon, catalog number: 1006090 )
Note: Currently, it is “Sigma-Aldrich, catalog number: 1006090”. - Mineral oil (Sigma-Aldrich, catalog number: M5904 )
- Paraformaldehyde (Sigma-Aldrich, catalog number: F8775 )
- 25% glutaraldehyde (Junsei, catalog number: 273721250 )
- Sodium carcodylate (Ted Pella, catalog number: 18851 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5379 )
- Ethanol (Merck Millipore Corporation, Calbiochem®, catalog number: 1.00983.1011 )
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 30620 )
- Osmium tetroxide (OsO4) (Ted Pella, catalog number: 96049 )
- Spurr resin (Ted Pella, catalog number: 183004221 )
- Double end mold (Ted Pella, catalog number: 10590 )
- Toluidine blue O (Sigma-Aldrich, catalog number: T3260 )
- EDTA (BioShop, catalog number: EDT001.500 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8532 )
Note: This product has been discontinued. - 4’, 6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, catalog number: 32670 )
- Acetic acid (Merck Millipore Corporation, Calbiochem®, catalog number: 1.00063.1011 )
- 1/2 strength Karnovsky’s fixative solution (see Recipes)
- Toluidine blue O solution (see Recipes)
- Fixative solution (see Recipes)
- DAPI solution for developing pollen (see Recipes)
- DAPI solution for mature pollen grain (see Recipes)
Equipment
- Ultramicrotome (Boeckeler Instruments, model: MT990 motorized precision microtome )
- Hot plate
- Water bath
- Incubator
- Fume hood
- Light and fluorescence microscope (Leica Microsystems, model: DM2500 )
- Vacuum pump and vacuum jar
- Glass pipette
Procedure
- Semi-thin sectioning of Arabidopsis floral buds for light microscopy
- Embedding of Arabidopsis floral buds
- Collect the whole inflorescence in a 2 ml tube.
- Add 1 ml of 1/2 strength Karnovsky’s fixative solution (Karnovsky, 1965) into the tube and cover it with 3MM Whatman filter paper beneath the surface of the solution.
- Incubate the tube in a vacuum jar under 400 mmHg for 30 min.
- Incubate the tube at 4 °C for 4 h to overnight.
- Wash the samples three times with 1 ml of 0.1 M sodium carcodylate (pH 7.4) for every 5 min on the rocker with gentle agitation.
- Carry out a post-fixation treatment for overnight at 4 °C with 1 ml of 1% osmium tetroxide using a glass pipette.
Note: If you want to do immuno-staining, do not perform post-fixation with osmium tetroxide. This can inhibit the binding of antibody to antigen. - Dehydrate the sample with 10, 20, 30, 40, 50, 60, 70, 80, and 90% ethanol for 30 min for each treatment sequentially and with 100% ethanol for overnight.
- Prepare medium spurr resin according to manufacturer’s protocol in the hood and then incubate the spurr resin in a vacuum jar for 30 min to eliminate air bubbles.
- Infiltrate the spurr resin prepared at step A1-h to the dehydrated sample at step A1-g and remove the residual ethanol of the sample by the following procedures.
Step 100 % Ethanol : Pure resin Time (h) ① 3 : 1 4 ② 1 : 1 4 ③ 1 : 3 4 ④ − : 4 Overnight - Fill the bottom of the double end mold tray with spurr resin, and then incubate it at 58 °C for overnight to make drop panel.
- Immerse the sample in the mold tray with spurr resin, and then incubate it at 58 °C for 3 days.
- Take out the block from the mold tray and keep the sample at room temperature.
- Collect the whole inflorescence in a 2 ml tube.
- Semi-thin sectioning of the sample embedded in the spurr resin.
- Trim the resin block ends with a razor blade to make the shape of trapezoid centering around a specimen (Figure 1).
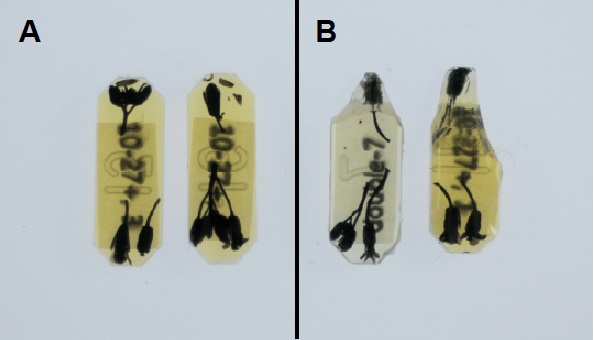
Figure 1. Resin blocks embedded specimens. A. Arabidopsis inflorescences embedded in solidified resin. B. Trimmed resin by razor blade. - Fix the resin block to the head of the microtome.
- Cut 1-10 μm transverse sections of the resin-embedded specimens using an MT-990 motorized precision microtome with the glass knives. Four μm transverse sections were made in the case of Borg et al. (2009).
- Collect the floating samples in water on to a glass slide with a platinum wire and put the glass slide with the samples on a hot plate at 50 °C for 5 min.
- Drop 100 μl of the toluidine blue solution on the surface of the sections by using a syringe, and then dry it on the hot plate at 50 °C for 5 min.
- Wash the samples with sprinkled water using a syringe, and then dry the excess moisture on the slide put on the hot plate at 50 °C.
- Drop 20 μl of the mineral oil and cover it with a coverslip, then seal the edges of a coverslip with nail varnish (Figure 2).
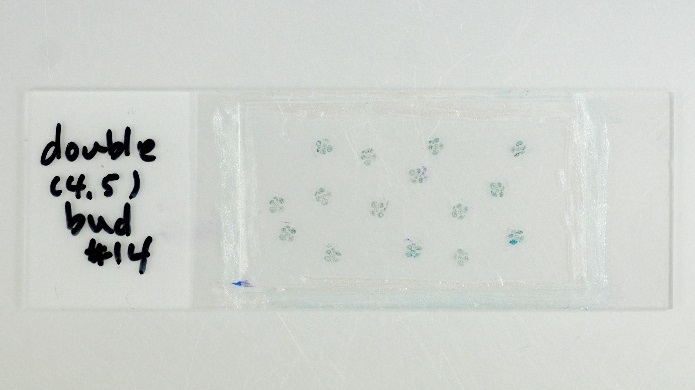
Figure 2. Sections stained with toluidine blue solution on a slide with a coverslip
- Trim the resin block ends with a razor blade to make the shape of trapezoid centering around a specimen (Figure 1).
- Embedding of Arabidopsis floral buds
- Fluorescence microscopic analysis of developing microspore and mature pollen with 4', 6-diamidino-2-phenylindole (DAPI)
- Preparation of mature pollen grains with DAPI staining.
- Fix the whole inflorescence with opened flowers in a fixative solution (Oh et al., 2010) for 5 min.
Note: If you want to detect fluorescent proteins or GUS staining, skip this procedure. - Collect 3-4 opened flowers at flower stage 13-14 from Arabidopsis (Sanders et al., 1999) in a 1.5 ml tube containing 200 μl of DAPI solution (Park et al., 1998).
- Vortex the tube briefly at maximum speed for 10 sec to separate pollen grains from the dehiscent anther wall.
- Incubate the tube in the dark at room temperature for 30 min.
- Transfer the supernatant to a new 1.5 ml tube with a 200 μl pipette man.
Note: Do not centrifuge. Do not transfer any other floral organs except pollen grains in the supernatant. - Concentrate the pollen grains by centrifuging at 13,000 rpm for 30 sec at room temperature, and then discard the supernatant.
- Resuspend the pollen pellets in 5-10 μl of DAPI solution.
- Mount this sample on the microscope slide under the coverslip.
- Fix the whole inflorescence with opened flowers in a fixative solution (Oh et al., 2010) for 5 min.
- Preparation of developing microspores from anthers with DAPI staining
- Fix the whole inflorescence with opened flowers in a fixative solution (Oh et al., 2010) for 5 min.
Note: If you want to detect fluorescent proteins or GUS staining, skip this procedure. - Dissect the single anther from inflorescence on a microscope slide with a needle while adding 4-5 μl of 0.3 M mannitol or DAPI solution (Park et al., 1998).
- Collect free microspores on a slide in a 1.5 ml tube and incubate them in the dark for 30 min.
- Mount this sample on the microscope slide under the coverslip.
- Fix the whole inflorescence with opened flowers in a fixative solution (Oh et al., 2010) for 5 min.
- Acquire images with a camera affixed to a Leica DM2500 microscope under the epifluorescence channel (15% of 405 nm/430-470 nm for DAPI, 470 nm/530 nm for GFP and 561 nm/570-610 nm for DsRed) (Figure 3) (Kim et al., 2015).
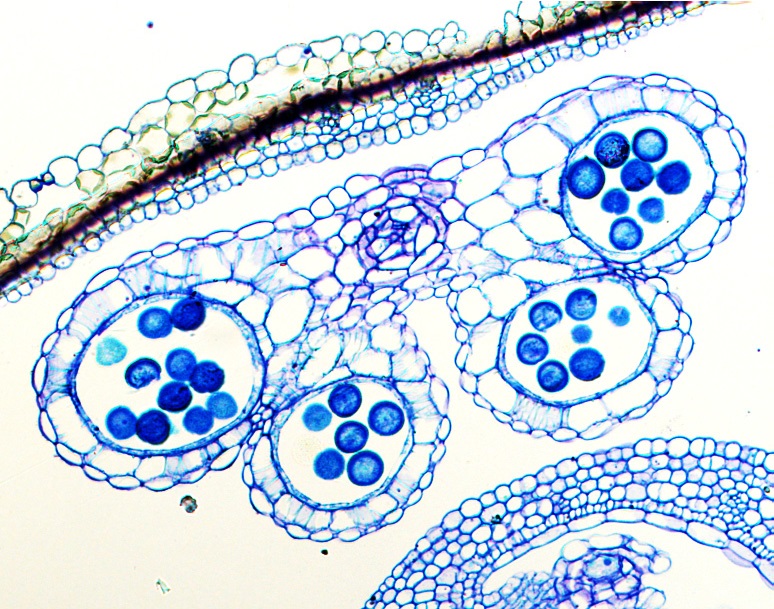
Figure 3. Image of transverse sections of Arabidopsis anther stained with toluidine blue solution by light microscopy according to the protocol
- Preparation of mature pollen grains with DAPI staining.
Recipes
- 1/2 strength Karnovsky’s fixative solution
2% paraformaldehyde
2.5% glutaraldehyde
0.1 M sodium cacodylate (pH 7.4)
Prepare this solution freshly prior to use - Toluidin blue O solution
Dissolve powder in 1 or 2 ml of water to make final 0.05% solution, and filter this solution through a 0.2 μm sterile syringe filter. Store this solution at 4 °C protected from light. - Fixative solution
Ethanol: acetic acid=3:1
Prepare this solution freshly prior to use. - DAPI solution for developing pollen
0.1 M sodium phosphate (pH 7.0)
1 mM EDTA (pH 8.0)
0.1% Triton X-100
1 μg/ml DAPI
Store this solution at 4 °C protected from light - DAPI solution for mature pollen grain
0.1 M sodium phosphate (pH 7.0)
1 mM EDTA, pH 8.0
0.1% Triton X-100
0.4 μg/ml DAPI
Store this solution at 4 °C protected from light
Acknowledgments
This study was supported by grants from the Next-Generation BioGreen 21 Program (PJ01104701), Rural Development Administration, Republic of Korea and Basic Science Research Programs through the National Research Foundation of Korea funded by the Ministry of Education (no. 2013R1A1A2062335). This protocol was based on Kim et al. (2015).
References
- Berger, F. and Twell, D. (2011). Germline specification and function in plants. Annu Rev Plant Biol 62: 461-484.
- Borg, M., Brownfield, L. and Twell, D. (2009). Male gametophyte development: a molecular perspective. J Exp Bot 60(5): 1465-1478.
- Karnovsky, M. J. (1965). A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27(2): 137A-138A.
- Kim, M. J., Kim, M., Lee, M. R., Park, S. K. and Kim, J. (2015). LATERAL ORGAN BOUNDARIES DOMAIN (LBD)10 interacts with SIDECAR POLLEN/LBD27 to control pollen development in Arabidopsis. Plant J 81(5): 794-809.
- McCormick, S. (2004). Control of male gametophyte development. Plant Cell 16 Suppl: S142-153.
- Oh, S. A., Park, K. S., Twell, D. and Park, S. K. (2010). The SIDECAR POLLEN gene encodes a microspore-specific LOB/AS2 domain protein required for the correct timing and orientation of asymmetric cell division. Plant J 64(5): 839-850.
- Park, S. K., Howden, R. and Twell, D. (1998). The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125(19): 3789-3799.
- Sanders, P. M., Bui, A. Q., Weterings, K., McIntire, K. N., Hsu, Y. C., Lee, P. Y., Truong, M. T., Beals, T. P. and Goldberg, R. B. (1999). Another developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11(6): 297-322.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kim, M. and Kim, J. (2016). Semi-thin Sectioning, Light and Fluorescence Microscopy of Floral Bud to Study Microspore Development in Arabidopsis. Bio-protocol 6(5): e1752. DOI: 10.21769/BioProtoc.1752.
Category
Plant Science > Plant developmental biology > Morphogenesis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











