- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
VAMP8-3xHA Uptake Assay in HeLa Cells
Published: Vol 6, Iss 4, Feb 20, 2016 DOI: 10.21769/BioProtoc.1739 Views: 8638
Reviewed by: Ralph BottcherVaibhav B ShahAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Optimizing Confocal Imaging Protocols for Muscle Fiber Typing in the Mouse Masseter Muscle
Catalina Matias [...] Jeffrey J. Brault
Apr 5, 2025 2918 Views

Development of a Novel Automated Workflow in Fiji ImageJ for Batch Analysis of Confocal Imaging Data to Quantify Protein Colocalization Using Manders Coefficient
Vikram Aditya [...] Wei Yue
Apr 5, 2025 2880 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 496 Views
Abstract
Transmembrane proteins are rarely exclusively localized to a specific vesicle or an organelle. Most transmembrane proteins undergo complicated trafficking routes. Thus, transmembrane proteins are under constant flux, and at steady state, found on a variety of vesicles or organelles. This characteristic makes the study of their trafficking routes complex, since at any given moment, different molecules are often being trafficked in opposing directions. Pulse-chase experiments can temporally track a specific pool of a transmembrane protein of interest, allowing for the kinetic description of its trafficking route. This type of technique has been used extensively to follow a large array of plasma membrane localized proteins (Diril et al., 2006; Jean et al., 2010). Here, we describe a method that allows the study of VAMP8 trafficking from the plasma membrane to endolysosomal compartments. This method was used to describe a role for MTMR13 and RAB21 in the regulation of VAMP8 trafficking to endolysosomes (Jean et al., 2015).
Keywords: Membrane traffickingMaterials and Reagents
- Costar® 24 Well Clear TC-Treated Well Plates (Corning, catalog number: 3526 )
- #1.5 round glass coverslip (Ted Pella, catalog number: 260368 )
- 100 mm Tissue Culture Dish (Corning, catalog number: 430167 )
- 0.22 µm filtering unit (Genesee Scientific Corporation, catalog number: 25-227 )
- HeLa cells (ATCC, catalog number: CCL-2 )
- JetPRIME (Polyplus-transfection, catalog number: 114-07 )
- pCDNA3-VAMP8-3xHA expression plasmid (self-made) (Jean et al., 2015)
- Dulbecco’s Modified Eagle Medium with High glucose with 4 mM L-Glutamine and sodium pyruvate (GE Healthcare, HycloneTM, catalog number: SH30243.FS ) (see Recipes for complete DMEM)
- Fetal Bovine Serum (Sigma-Aldrich, catalog number: F2442-500 ml )
- Penicillin-Streptomycin solution (Life Technologies, catalog number: 15140-122 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 15140-122”. - Trypsin-EDTA (0.25%) (Life Technologies, catalog number: 25200-056 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 25200-056”. - 0.4% Trypan-Blue (Life Technologies, catalog number: 15250-061 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 15250-061”. - Rabbit anti-HA antibody (Abcam, catalog number: ab9110 )
- Earl’s Balanced Salt Solution (with sodium bicarbonate, without phenol red, liquid, sterile- filtered) (Sigma-Aldrich, catalog number: E3024-500 ml )
- Bovine Serum Albumin (Fraction V, Heat shock treated) (Thermo Fisher Scientific, catalog number: BP1600-100 )
- Sodium Chloride (NaCl) (Thermo Fisher Scientific, catalog number: S671-3 )
- Potassium Chloride (KCl) (VWR International, catalog number: BDH9258-500 g )
- Sodium Phosphate Dibasic Anhydrous (Na2HPO4) (Thermo Fisher Scientific, catalog number: S374500 )
- Potassium Phosphate Monobasic (KH2PO4) (Thermo Fisher Scientific, catalog number: P285-S500 )
- Paraformaldehyde (Thermo Fisher Scientific, catalog number: AC41678-5000 )
- Goat Serum (Life Technologies, catalog number: 16210-064 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 16210-064”. - Triton X-100 (Sigma-Aldrich, catalog number: X100-500 ml )
- Mouse anti-human Lamp1 (Developmental Studies Hybridoma Bank, catalog number: H4A3 )
- Mouse anti-EEA1 (BD Biosciences, catalog number: 610457 )
- Goat anti-Mouse IgG (H+L) Secondary Antibody, Alexa Fluor® 488 conjugate (Life Technologies, catalog number: A-11029 )
Note: Currently, it is “Thermo Fisher Scientific, InvitrogenTM, catalog number: A-11029”. - Goat anti-Rabbit IgG (H+L) Secondary Antibody, Alexa Fluor® 546 conjugate (Life Technologies, catalog number: A11035 )
Note: Currently, it is “Thermo Fisher Scientific, InvitrogenTM, catalog number: A11035”. - [4’, 6-Diamidino-2-Phenylindole, Dihydrochloride] (DAPI) (Life Technologies, catalog number: D1306 )
Note: Currently, it is “Thermo Fisher Scientific, Molecular ProbesTM, catalog number: D1306”. - FluorSave reagent (Merck Millipore Corporation, Calbiochem®, catalog number: 345789 )
- Complete DMEM (see Recipes)
- 1x Phosphate Buffer Saline (PBS) (see Recipes)
- 4% Paraformaldehyde solution (see Recipes)
- Blocking buffer (see Recipes)
- Antibody incubation buffer (see Recipes)
Equipment
- 37 °C, 5% CO2 cell culture incubator [similar to Thermo Scientific Heracell VIOS 160i CO2 incubator (Thermo Fisher Scientific, catalog number: 51030285 )]
- Tissue culture biosafety cabinet [similar to Thermo Scientific 1300 Series Class II (Thermo Fisher Scientific, catalog number: 1323)]
- Hemacytometer (VWR International, catalog number: 100498-470 )
- Pipettes 10 μl, 100 μl and 1,000 μl (Eppendorf, catalog numbers: 3120000020 , 3120000046 and 31200000623 )
- Vacuum Flask (self-made)
Note: Required to remove media from the 24 well plates during the immunofluorescence protocol. - Microcentrifuge (Eppendorf, catalog number: 5415D )
Procedure
- Day 1: Seeding cells
- Add 1 circular sterile coverglass per well of a 24 well plate. Use sterile forceps to manipulate cover glasses. (The number of wells used depends on the number of samples and time points). Coverglasses are sterilized by autoclaving in a glass dish for 30 min.
- Remove media from 100 mm stock HeLa plate, and wash with 5 ml of 1x PBS. Either room temperature or 37 °C pre-warmed PBS is suitable for the steps listed in procedure A.
- Remove PBS, add 1 ml of 0.25% trypsin-EDTA, and incubate at 37 °C for 3 min.
- Wash cells off the plate with 10 ml of complete DMEM.
- Pipet 20 μl of the cell suspension, and add to 100 μl of Trypan-Blue solution in an Eppendorf tube.
- Pipet 10 μl of this cell/Trypan-Blue dilution into a hemacytometer chamber.
- Count viable cells using the hemacytometer on a standard microscope. Viable cells will be Trypan-Blue negative.
- Add a total of 1.5 x 104 cells per well of a 24-well plate.
- Bring to 1.5 ml of complete DMEM per well, and move to 37 °C incubator overnight (o/n).
- Add 1 circular sterile coverglass per well of a 24 well plate. Use sterile forceps to manipulate cover glasses. (The number of wells used depends on the number of samples and time points). Coverglasses are sterilized by autoclaving in a glass dish for 30 min.
- Day 2: HeLa cells Transfection using JetPrime
- Remove media from each well and replace with 0.5 ml of complete DMEM.
- In a 1.5 ml Eppendorf tube, add 500 ng of pCDNA3-VAMP8-3xHA and adjust to a final volume of 100 μl with JetPrime buffer. The amount of DNA, JetPrime buffer and JetPrime mentioned are sufficient for the transfection of 6 wells of a 24 well plate. This can be scaled up or down depending on the number of wells needed.
- Vortex for 10 sec, and spin down in a microcentrifuge at 1,000 rpm for 5 sec.
- Add 2 μl of JetPrime transfection reagent.
- Vortex for 10 sec, and spin down as in step B3.
- Incubate at room temperature for at least 10 min.
- Add 15 μl of transfection mix per well. Each master mix can be used on 6 wells.
- Incubate 6 hours at 37 °C in the tissue culture incubator.
- Remove the media and replace with 1.5 ml of complete DMEM.
- Incubate overnight in the 37 °C tissue culture incubator.
- Remove media from each well and replace with 0.5 ml of complete DMEM.
- Day 3: VAMP8 endocytic uptake assay
- Wash transfected cells with 1 ml of ice-cold DMEM. Repeat one more time for a total of two washes. Remove all the media.
- Dilute rabbit anti-HA antibody in ice cold complete DMEM to a final dilution of 1:200.
- Add 150 μl of diluted anti-HA antibody to each well.
- Incubate on ice for 60 min with occasional rocking. (This step allows binding of the antibody
to plasma membrane-localized VAMP8).
- Wash cells three times with 1ml of ice-cold complete DMEM. (This step removes all unbound antibodies).
- Wash cells two times with 1 ml of pre-warmed EBSS (37 °C).
- Add 1 ml of pre-warmed EBSS to each well, and incubate in the 37 °C tissue culture incubator for 15, 45 and 90 min. Please refer to the section, 'Important experimental controls for uptake assay validation,' in order to prepare all the necessary controls.
- Wash transfected cells with 1 ml of ice-cold DMEM. Repeat one more time for a total of two washes. Remove all the media.
- Immunofluorescence (Day 3 and 4)
- At each time point, remove the appropriate coverglass and transfer into a new well of a new 24-well plate containing 1 ml of 1x PBS.
- Remove the 1x PBS, and replace with 1 ml of the 4% paraformaldehyde solution.
- Incubate for 15 min at room temperature.
- Remove 4% paraformaldehyde solution, and wash three times with 1 ml of 1x PBS. For each wash, incubate 5 min at room temperature.
- Remove last wash and block in 1 ml of blocking buffer for 1 h at room temperature.
- Dilute mouse anti-Lamp1 or mouse anti-EEA1 to final dilutions of 1:100 or 1:1,000, respectively, in antibody incubation buffer.
- Remove blocking buffer and replace with 100 μl of the appropriately diluted antibody, and incubate at 4 °C o/n.
- Remove diluted antibody solution, and wash 3 times with 1 ml of 1x PBS. For each wash, incubate 5 min at room temperature.
- Dilute Alexa-coupled secondary antibodies. Dilute goat anti-mouse Alexa-488 and goat anti-rabbit Alexa-546 to a final dilution of 1:250 in antibody dilution buffer.
- Remove wash solution, add 200 μl of diluted secondary antibody solution, and incubate 1 h
at room temperature in the dark to minimize photobleaching.
- Remove secondary antibody-containing solution, and wash three times with 1 ml of 1x PBS.
For each wash, incubate 5 min at room temperature.
- Add 200 μl of 1x PBS containing 1 μg/ml of DAPI and incubate 5 min at room temperature.
- Remove DAPI-containing solution and wash twice with 1 ml of 1x PBS. For each wash, incubate 5 min at room temperature.
- Pipet 10 μl of FluorSave mounting media on a microscope slide. Remove coverglass from individual wells using forceps, and gently remove as much liquid as possible. Gently drop the coverglass onto the slide and the mounting media, making sure that no air bubbles are trapped between the coverglass and the slide. Remove extra mounting solution, and let the slide cure o/n at 4 °C.
- Image on an appropriate confocal or widefield microscopy system.
- At each time point, remove the appropriate coverglass and transfer into a new well of a new 24-well plate containing 1 ml of 1x PBS.
- Automated quantification of colocalization
- Export microscope images to 16 bit tiff files.
- Use CellProfiler software to analyze colocalization (http://www.cellprofiler.org).
- Using CellProfiler, generate a pipeline that allows the identification of cell boundaries and of the different objects: EEA1 vesicles, LAMP1 vesicles and VAMP8 vesicles. See Jean et al. (2015) for detailed methods.
- Measure object colocalization by following the recommendations found at http://www.cellprofiler.org/linked_files/ExampleColocalization_Tutorial.pdf).
- Once the number of colocalized objects is established, correlate the percentage of colocalized objects per cells using excel.
Important experimental controls for uptake assay validation
In order to insure valid and interpretable endocytic uptake experiments, the following controls should be performed:
Validate that the antibody labels only surface localized proteins at time 0.- Include a condition that is held only at 4 °C for the antibody binding step but not permitted to undergo uptake at 37 °C. Perform the immunofluorescence as described for other timepoints with uptake conditions. Staining should only be localized around the plasma membrane, which will be evident when analyzed by
confocal microscopy.
- Given that most uptake experiments rely on transient transfection and detection of an epitope tag, a mixed population of cells will be present in the dish. Thus, in line with transfection efficiency, untransfected cells should not display any staining.
- Perform a 4 °C incubation in the absence of chase antibody (HA antibody used to label VAMP8:3xHA) and fix the cells. Stain cells using a conventional immunofluorescence protocol (with primary HA antibody and secondary antibodies Goat anti-rabbit Alexa-546) to identify the steady-state localization of the protein of interest. For many proteins, the total protein staining at steady-state typically detects signal mainly on internal punctae, reflecting protein trafficking through internal compartments.
In conclusion, the lack of plasma membrane staining in untransfected cells together with exclusive plasma membrane staining in transfected cells at time 0, coupled with a punctate pattern in permeabilized cells, argues for staining specificity. Rapid endocytosis of chased proteins (VAMP8:3xHA) further indicates a functional uptake assay. Altogether, these results validate the endocytic uptake assay. Note that this assay requires additional modifications to monitor recycling of surface-labeled cargo to the plasma membrane (van Weert et al., 2000).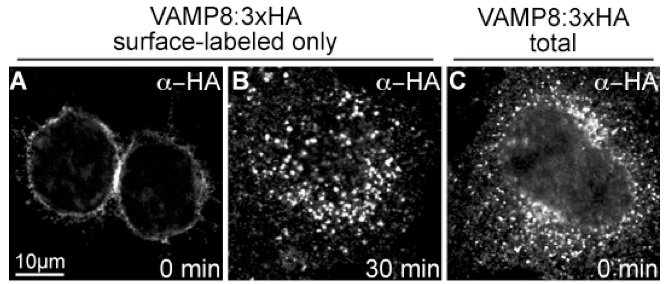
Figure 1. Control experiments to insure a functional uptake assay. A. Cells labeled with anti-HA antibody and without a 37 °C incubation show only surface membrane staining, while (B) cells chased at 37 °C for 30 min show intracellular staining. C. Total VAMP8:3xHA detected by regular immunofluorescence shows mostly an intracellular pattern.
- Export microscope images to 16 bit tiff files.
Recipes
- Complete DMEM
Remove 55 ml of DMEM from the purchased 500 ml HyClone bottle
Add 50 ml of fetal bovine serum (yields a final concentration of 10%)
Add 5 ml of Penicillin/Streptomycin solution (yields a final concentration of 1%)
Filter sterilized with filtering device (0.2 μm) - 1x phosphate buffer saline (PBS)
Mix 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4 and 0.24 g of KH2PO4
Add 900 ml of dH2O
Adjust pH to 7.4
Adjust final volume to 1,000 ml
Sterilized by autoclaving at 121 °C for 20 min - 4% paraformaldehyde solution
Add 4 g of paraformaldehyde to 80 ml of 1x PBS
Heat to 65 °C (in a water bath) with occasional vortexing
Adjust pH to 7.4 with NaOH
Complete to 100 ml with 1x PBS
Filter sterilize (0.2 μm)
Use fresh - Blocking buffer
Mix 5 ml of Goat serum to 95 ml of 1x PBS (yields a final concentration of 5%)
Add 0.3 ml of Triton X-100 (Use a wide bore tip to pipette Triton X-100) (yields a final concentration of 0.3%)
Mix well - Antibody incubation buffer
Add 0.1 g of BSA to 10 ml of 1x PBS (yields a final concentration of 1%)
Add 0.03 ml of Triton X-100 (Use a wide bore tip to pipette Triton X-100) (yields a final concentration of 0.3%)
Mix well
Acknowledgments
The uptake assay was adapted from the previously published study (Miller et al., 2011) and was performed in (Jean et al., 2015). The immunofluorescence protocol was adapted from Cell Signaling Technology, http://www.cellsignal.com/common/content/content.jsp?id=if. This work was supported by FRSQ, AHA and CRS postdoctoral fellowships to SJ, and NIH RO1 GM078176 and support from the SDCSB NIH P50 GM085764 to AAK.
References
- Diril, M. K., Wienisch, M., Jung, N., Klingauf, J. and Haucke, V. (2006). Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell 10(2): 233-244.
- Jean, S., Cox, S., Nassari, S. and Kiger, A. A. (2015). Starvation-induced MTMR13 and RAB21 activity regulates VAMP8 to promote autophagosome-lysosome fusion. EMBO Rep 16(3): 297-311.
- Jean, S., Mikryukov, A., Tremblay, M. G., Baril, J., Guillou, F., Bellenfant, S. and Moss, T. (2010). Extended-synaptotagmin-2 mediates FGF receptor endocytosis and ERK activation in vivo. Dev Cell 19(3): 426-439.
- Miller, S. E., Sahlender, D. A., Graham, S. C., Honing, S., Robinson, M. S., Peden, A. A. and Owen, D. J. (2011). The molecular basis for the endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell 147(5): 1118-1131.
- van Weert, A. W., Geuze, H. J., Groothuis, B. and Stoorvogel, W. (2000). Primaquine interferes with membrane recycling from endosomes to the plasma membrane through a direct interaction with endosomes which does not involve neutralisation of endosomal pH nor osmotic swelling of endosomes. Eur J Cell Biol 79(6): 394-399.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Jean, S. and Kiger, A. A. (2016). VAMP8-3xHA Uptake Assay in HeLa Cells. Bio-protocol 6(4): e1739. DOI: 10.21769/BioProtoc.1739.
- Jean, S., Cox, S., Nassari, S. and Kiger, A. A. (2015). Starvation-induced MTMR13 and RAB21 activity regulates VAMP8 to promote autophagosome-lysosome fusion. EMBO Rep 16(3): 297-311.
Category
Cell Biology > Cell-based analysis > Transport
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











