- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mouse BMDC-dependent T Cell Polarization Assays
Published: Vol 6, Iss 3, Feb 5, 2016 DOI: 10.21769/BioProtoc.1721 Views: 15863
Reviewed by: Fanglian HeMeenal SinhaMarco Di Gioia

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Functional Phenotyping of Lung Mouse CD4+ T Cells Using Multiparametric Flow Cytometry Analysis
Céline M. Maquet [...] Bénédicte D. Machiels
Sep 20, 2023 4230 Views

T-Cell-Based Platform for Functional Screening of T-Cell Receptors Identified in Single-Cell RNA Sequencing Data Sets of Tumor-Infiltrating T-Cells
Aaron Rodriguez Ehrenfried [...] Rienk Offringa
Apr 20, 2024 6427 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3576 Views
Abstract
In response to exposure to antigen, T cells whose T cell receptor (TCR) are capable of recognizing the self MHC-antigen derived peptide complex, respond to the antigen and differentiate into one of several subsets, namely TH1, TH2, TH17, Treg, etc. characterized by the signature cytokine they secrete, namely IFN-γ, IL-4, IL-17 or IL-10, respectively, referred to as syngeneic polarization as the MHC presenting the foreign antigen/epitope is self-derived.
T cell responses following incubation for defined periods, usually 3 days for mouse splenocytes, are routinely measured by assessing the antigen-stimulated proliferation of T cells by measuring the radiolabeled precursor thymidine incorporated into the genomic DNA of the dividing T cell; the direction of polarization is assessed by measuring the cytokine produced by the proliferating or non-proliferating responding T cells using ELISA of culture supernatants or by intracellular cytokine staining followed by flow cytometry.
In the protocols detailed below, we describe the use of syngeneic mouse bone marrow-derived primary dendritic cells (BMDC) as APC to stimulate spleen derived T cells. The proliferative response of the T cells is measured by incorporation of radiolabeled precursor thymidine into the genomic DNA and their direction of polarization is assessed by measuring the cytokines they secrete, namely IFN-γ, IL-4 and IL-17 over a 72 h period using ELISA. In addition, we used flow cytometry after intracellular cytokine staining to detect IL-17 positive T cells within the CD3+/CD4+/CD25low population. Prior live infection of BMDC with strains of Mycobacterium bovis- Bacille Calmette Guerin (BCG) was used as antigen to pre-condition the BMDC that presented antigens derived therefrom to T cells. We also measured cytokines secreted within 6 to 8 h of BCG infection by BMDC in order to correlate the BMDC cytokine profile with subsequent direction of T cell polarization.
Materials and Reagents
- 10 cm Petri dishes, non-treated (Thermo Fisher Scientific, FalconTM, catalog number: 08-757-100D )
- 6 well 35 mm dia plastic cell culture treated dishes (Thermo Fisher Scientific, FalconTM, catalog number: 353046 )
- 0.22 µm filter
- BALB/c mice (6 to 8 weeks old female)
- Iscove’s modification of Dulbecco’s minimum essential medium (IMDM) (Life Technologies, catalog number: 12200069 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 12200069”. - Sterile PBS
- Dulbecco’s Phosphate buffered saline (Sigma-Aldrich, catalog number: D5773 )
- Saponin from quillaja bark (wash buffer for flow cytometry staining) (Sigma-Aldrich, catalog number: S7900 )
- Murine rGM-CSF (recombinant mouse GM-CSF) (PEPROTECH, catalog number: 315-03 )
Note: Recombinant GM-CSF from Peprotech Reconstitute powder (recommended by manufacturer). - ELISA antibodies
Note: We use R&D Systems Duoset capture and detection antibodies or BD paired ELISA antibody sets as detailed below:
Mouse IFN-γ DuoSet ELISA (R&D Systems, catalog number: DY485-05 )
Mouse IL-17 DuoSet ELISA (R&D Systems, catalog number: DY421-05 )
Mouse IL-6 DuoSet ELISA (R&D Systems, catalog number: DY406-05 )
Mouse TNF-α DuoSet ELISA (R&D Systems, catalog number: DY410-05 )
Mouse IL-10 DuoSet ELISA (R&D Systems, catalog number: DY417-05 )
Mouse IL-2 DuoSet ELISA (R&D Systems, catalog number: DY402-05 )
Mouse IL-12p40 DuoSet ELISA (R&D Systems, catalog number: DY499-05 )
Mouse IFN-gamma DuoSet ELISA (R&D Systems, catalog number: DY1679-05 ) - 1x PBS-1% BSA-0.05% sodium azide
- Concanavalin A from Canavalia ensiformis (Jack bean) (Sigma-Aldrich, catalog number: C5275 )
- CD11c MicroBeads (Miltenyi Biotech, catalog number: 130-052-001 )
- Glutamax-100x (Thermo Fisher Scientific, GibcoTM, catalog number: 35050-061 )
- Mitomycin C from Streptomyces caespitosus (Sigma-Aldrich, catalog number: M4287 )
- Penicillin-Streptomycin, 100x (Thermo Fischer Scientific, GibcoTM, catalog number: 15070-063 )
- Penicillin-Streptomycin (10,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140-122 )
- IL-17-APC (eBioscience, clone: eBio17B7 )
- 10% FBS
- [methyl-3H]
- 70% and 100% ethanol
- Brefeldin A (BFA) (Sigma-Aldrich, catalog number: B7651 )
- Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: A9434 )
- Potassium bicarbonate (KHCO3) (Sigma-Aldrich, catalog number: 60339 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E6758 )
- Tris (pH 7.5)
- IMDM complete (see Recipes)
- Thymidine (see Recipes)
- Brefeldin A (BFA) (Sigma-Aldrich, catalog number: B7651) (see Recipes)
- Monensin sodium salt (Sigma-Aldrich, catalog number: M5273 ) (see Recipes)
- RBC lysis buffer (100 ml) (see Recipes)
- RBC lysis buffer (see Recipes)
Equipment
- Sterile scissors and forceps
- Cell scrapers (Corning, Costar®, model: 3010 Small Cell Scraper )
- Centrifuges (Table top) (Eppendorf)
- Water bath set at 37 °C
- Flow cytometer [FACS-Canto-II flow cytometer (BD) or any machine with Argon ion Blue laser)
- Biosafety cabinet with laminar air flow
Procedure
Note: The protocol outlined below has been adapted from Satchidanandam et al. (2014).
- Bone marrow cell isolation for BMDC culture (all steps to be performed at room temperature) (Figure 1)
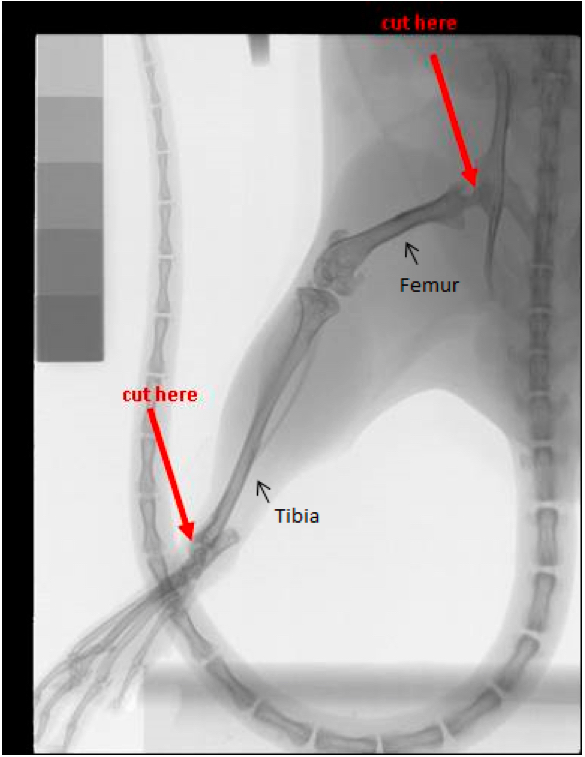
Figure 1. Bone marrow cell isolation for BMDC culture. The figure shows the X-ray picture of a mouse hind limb. The femur and tibia are labelled, The points where the bones have to be cut during the initial dissection are clearly marked by arrows.
.- We used female BALB/c mice bred in-house in the Institute’s central animal facility. Use of mice (and any other animals) requires prior approval by the Institutional Animal Ethics Committee. The studies were carried out in strict accordance with the Institutional Animal Ethics Committee-approved protocols of the Indian Institute of Science for mouse experiments (CAF/Ethics/220-2011 dated February 10, 2011). For details of Animal Ethics guidelines, please refer to Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines (https://www.nc3rs.org.uk/arrive-guidelines). Mice were administered inhalation anesthesia with chloroform and euthanized by cervical dislocation.
- Handle the mice gently and avoid making the animals edgy before they are sacrificed.
- Using dissection tweezers and dissection scissors remove the skin on the lower abdomen and both legs (Video 1). This will expose both legs from the hip down to the metatarsals. Proper removal of the skin at this point will prevent contamination of cultures with epidermal cells. Begin by separating the muscle along the entire leg from the skin. Then proceed by using the tweezers to stabilize the leg while removing the muscle with scissors and scalpel blade.
Video 1. Dissect mouse leg - Remove the femur and tibia as follows:
- Make one cut at the hip. Be sure to keep as much of the epiphysis intact as possible on the femur. To do this, position the scissors as close to the hip as possible while cutting (Figure 1).
- Make the second cut below the ankle joint, again being careful to leave the epiphysis intact (Figure 1).
- Place the harvested femur and tibia (should still be connected at the knee-joint) in a sterile Petri plate in about 5 ml of IMDM containing Penicillin and Streptomycin. Use bacteriological Petri dishes with lid.
- Make one cut at the hip. Be sure to keep as much of the epiphysis intact as possible on the femur. To do this, position the scissors as close to the hip as possible while cutting (Figure 1).
- Remove all the flesh clinging to the bones using a scalpel blade, scissors and forceps (Video 2).
Video 2. Clean bones - Transfer bones to a new Petri dish with fresh medium with antibiotics. Repeat removing flesh sticking to bones if necessary.
- Collect bone marrow under sterile conditions (Video 3).
Video 3. Flush bone marrow- Using a scissor first find the site of intersection between the femur and tibia and then very gently separate them from each other. Avoid exposing marrow by cutting off either femur or tibia during this process.
- For flushing out the marrow use different size needles for femur and tibia. For tibia use a 26 or 24 gauge needle and for femur-use a 21 gauge needle.
- Fill a sterile 5 ml syringe with about 5 ml of IMDM. Place a 26 gauge needle on the end of the syringe and keep ready for the next step. Prepare a fresh sterile non-treated Petri-dish to collect flushed bone marrow in the next step.
- Hold the bone with sterile tweezers and using a scissor gently pull each epiphysis off or cut off epiphysis to expose the bone marrow. (Avoid placing the cut bone back into the Petri place after cutting to avoid loss/leaching out of bone marrow.)
- Hold the cut bone over a sterile Petri dish and puncture the centre of the bone ends with the needle. It should slide in easily if it is in the center. Slowly flush out the bone marrow with sterile IMDM + antibiotics. If necessary, move the needle up and down in the bone. Repeat this process at the other end of the bone if necessary. Do not break the bone. The bone should appear white or translucent when it is clean.
- Repeat this procedure for the other bones. Do not use medium containing flushed marrow cells to repeat flushing of bones. Always suck up fresh medium into the syringe for each flush.
- Using a scissor first find the site of intersection between the femur and tibia and then very gently separate them from each other. Avoid exposing marrow by cutting off either femur or tibia during this process.
- Discard each bone once clean.
- Ensure that the bones are always kept submerged under complete IMDM as you handle one bone at a time. To cut open the ends of the bones, use a good sharp pair of scissors and avoid generating splinters. These fine bone pieces are extremely difficult to separate from the cells of the marrow. The differentiating BMDC will engulf them and undergo premature activation.
- Once the clumps of cells have been suspended by repeated trituration with a pipette, transfer the cells to a sterile 50 ml conical tube. Let the cell suspension in a volume of about 40 ml, stand in a conical 50 ml tube for 5 min. Use a pipette to aspirate the top 35 ml, leaving behind debris and cell clumps. These clumps can be once again triturated to obtain more cells in suspension. You may alternately filter the cell suspension through a 70 µm filter to remove clumps.
- We used female BALB/c mice bred in-house in the Institute’s central animal facility. Use of mice (and any other animals) requires prior approval by the Institutional Animal Ethics Committee. The studies were carried out in strict accordance with the Institutional Animal Ethics Committee-approved protocols of the Indian Institute of Science for mouse experiments (CAF/Ethics/220-2011 dated February 10, 2011). For details of Animal Ethics guidelines, please refer to Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines (https://www.nc3rs.org.uk/arrive-guidelines). Mice were administered inhalation anesthesia with chloroform and euthanized by cervical dislocation.
- BMDC culture
- RBC Lysis. This step is extremely critical and should be carried out with great care. Excessive exposure to the lysis buffer can kill all cells.
- Spin cells at 1,000 x g, 10 min at room temperature (RT).
Collect supernatant after centrifugation in a clean tube. Do not discard. Check this supernatant for cells. This should not have more than a total of 2-4 million cells. - Very gently dislodge cell pellet into a thick slurry by gentle repeated tapping. Add 1 ml RBC lysis buffer for 50 million cells. Mix well to suspend the cells by gentle tapping. Incubate at RT, exactly 1 min 30 sec.
- Rapidly bring total volume up to 25 ml (add 24 ml) with plain IMDM and spin at 1,000 x g, 15 min at RT.
- Decant supernatant, gently re-suspend cell pellet and add fresh 20 ml IMDM, mix well to wash off any traces of RBC lysis buffer, and pellet cells once again at 1,000 x g, 15 min at RT.
Decant supernatant, gently re-suspend cell pellet and add 10 ml complete IMDM without GMCSF. Usually, from one mouse, total bone marrow cell yield is 40-70 million cells. Count cells and increase volume if required.
- Spin cells at 1,000 x g, 10 min at room temperature (RT).
- Bone marrow cells were cultured in IMDM-10% FBS containing 2 mM β-mercapto-ethanol essentially according to standard published protocols with 50 U/ml murine rGM-CSF. Some protocols call for 200 to 800 U/ml GM-CSF. We have found these high concentrations give rise to mature BMDC with high surface expression of MHCII and CD86. Standard antibiotics Penicillin-Streptomycin (10,000 U/ml) may be included at recommended concentrations. Seed 0.5 to 1.0 million cells per 35 mm dish in a volume of 3 ml.
- 50% of the culture medium was removed and replenished every two days.
- Non-adherent cells were harvested on day 7. One can get 1 to 2 million cells for every million cells seeded on day 0.
- Harvested cells were analysed by flow cytometry using phycoerythrin (PE)-conjugated antibodies against MHC-II, CD80 and CD86, individually in combination with FITC conjugated anti-mouse CD11c antibody to determine the percentage of immature dendritic cells which were then used for all experiments. A simple flow cytometer with an Argon ion (blue) laser will suffice to detect these two fluors. In mice, all subsets of DC express CD11c. Immature dendritic cells will express intermediate levels of MHC-II, CD80 and CD86. In most preparations, a small proportion, about 2 to 5% of the BMDC by day 7 will also express high levels of these co-stimulatory molecules. We normally obtain 60 to 85% cells being CD11c-positive by day 7. When high purity is required, we purify the CD11c-positive cells using mouse CD11c microbeads from Miltenyi Biotech according to manufacturer’s instructions.
- The BMDC thus obtained were infected at 5 multiplicity of infection (MOI) with Mycobacterium bovis BCG for 24 h. BMDC may also be incubated with other protein or peptide antigens; optimum incubation times required will need to be determined for each antigen.
- RBC Lysis. This step is extremely critical and should be carried out with great care. Excessive exposure to the lysis buffer can kill all cells.
- T cell polarization assay
- Splenocytes were isolated from BCG-immunized mice by dissecting the abdomen and removing the spleen within the biosafety cabinet to maintain sterility. The splenocyte suspension is prepared by forcing IMDM into the spleen tissue using a 20 ml syringe and 21 gauge needle. You may also gently press down on the spleen a few times using the flat rear end of a sterile syringe to release splenocytes into the surrounding medium. After counting the cell suspension, splenocytes are collected by centrifugation of the suspension at 1,300 x g for 10 min. RBC lysis was carried out for splenocytes exactly as described for bone marrow cells.
- 20 million splenocytes were then incubated in 10 ml complete IMDM for 2 h at 37 °C in 10 cm diameter cell culture dishes. Non-adherent cells were then collected for the assay.
- For syngeneic polarization studies, the above mentioned infected BMDC were killed by 50 µg/ml mitomycin C treatment for 1 h followed by extensive washing. BMDC were co-cultured in 200 µl volume in 96 well plates in triplicate with 5 x 105 non-adherent splenocytes per well from BALB/c mice infected intraperitoneally 3 weeks previously with 5 x 108 Mycobacterium bovis-BCG, at varying BMDC: splenocyte ratios of 1:10, 1:20 and 1:100 for 72 h. You may alternately kill infected BMDC by gamma irradiation (15 Gray) if you have access to this machine. Place the harvested BMDC in complete IMDM in a round bottom sterile snap cap tube and place inside the gamma irradiator. Control uninfected BMDC were also cultured with splenocytes similarly.
- After 72 h of co-culture of non-adherent splenocytes and killed BMDC, supernatants were collected for cytokine measurements. Identity of cytokine secreting cells can be determined by intracellular cytokine staining and flow cytometry. We routinely measured by ELISA, the cytokines IFN-γ, IL-4 and IL-17 to detect polarization of T cells to give TH1, TH2 or TH17 cells, respectively.
- Con-A stimulation of non-adherent splenocytes at 5 µg/ml was included as positive control.
- Splenocytes were isolated from BCG-immunized mice by dissecting the abdomen and removing the spleen within the biosafety cabinet to maintain sterility. The splenocyte suspension is prepared by forcing IMDM into the spleen tissue using a 20 ml syringe and 21 gauge needle. You may also gently press down on the spleen a few times using the flat rear end of a sterile syringe to release splenocytes into the surrounding medium. After counting the cell suspension, splenocytes are collected by centrifugation of the suspension at 1,300 x g for 10 min. RBC lysis was carried out for splenocytes exactly as described for bone marrow cells.
- Intracellular cytokine-staining and flow cytometry to detect TH17 cells
- 106 non-adherent splenocytes were cultured with 104 or 5 x 103 irradiated BMDC In 24 well tissue culture plates in triplicate wells.
- 10 h later, 10 µg/ml brefeldin A and 0.75 µM monensin were added for the next 26 h of culture. At the end of 36 h, harvested splenocytes were washed once with PBS-0.05% azide, and surface stained for CD4 (CD4-PE; clone GK1.5), CD25-PE-Cy7 (clone PC 61.5) and CD3-FITC (clone 145-2C-11) from BD Biosciences in PBS-1% BSA, washed with PBS-0.05% azide, fixed for 10 min. with 2% paraformaldehyde in 1x PBS and permeabilized in 500 µl of PBS-0.05% sodium azide -1% BSA with 0.1% saponin for 30 min.
- Intracellular IL-17 was detected using, IL-17-APC in PBS-0.05% N3-1% BSA with 0.1% saponin for 30 min on ice.
- Stained cells were washed once with 1 ml PBS-0.05% sodium azide - with 0.1% saponin.
- Stained and washed cell pellets were suspended in 100 µl PBS-0.05% sodium azide containing 1% paraformaldehyde, held on ice for 15 min. and diluted to 400 µl with PBS-0.05% azide.
- Samples were acquired on a BD FACS-Canto-II flow cytometer. Any flow cytometer with a blue (argon ion, 488 nm) and red (633 nm) laser will be suitable to detect this combination of fluors.
- IL-17 producing cells within the CD3-high, CD4-high and CD25-low population were enumerated.
- Identity of IL-17 producing cells was confirmed by using an isotype control antibody and more importantly, by staining with a fluorescence minus one (FMO) control missing the IL-17-APC antibody.
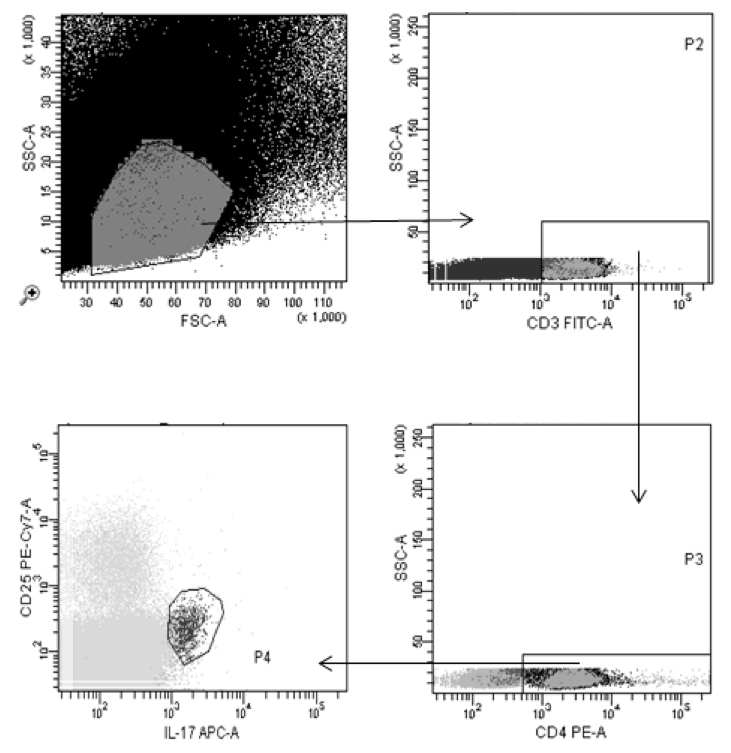
Figure 2. Gating strategy to detect IL-17 secreting T cells. Splenocytes stained as described were displayed on a linear FSC vs. SSC plot and the gate shown was applied to pick up all lymphocytes. Cells staining positive for CD3 from within this population were further selected and displayed for IL-17 and CD25. IL-17-positive cells within the CD25-low subset represent the true TH-17 subset of T cells.
- 106 non-adherent splenocytes were cultured with 104 or 5 x 103 irradiated BMDC In 24 well tissue culture plates in triplicate wells.
Representative data
Table 1. The table shows the levels of IFN-γ and IL-17 detected by ELISA in the culture supernatants of splenocytes co-cultured with the indicated BMDC samples. The cytokines are shown as pg/ml culture volume secreted by one million non-adherent splenocytes. 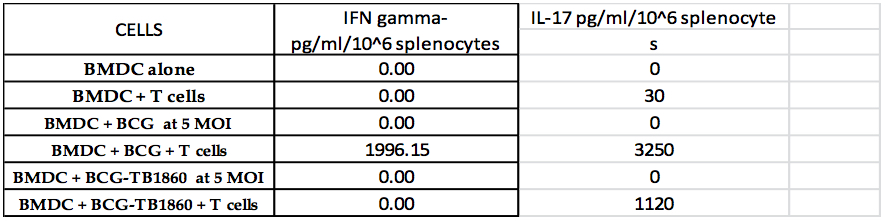
Notes
- Use of infection with live BCG strains to condition antigen presenting BMDC. BCG ensures robust stimulation of BMDC to produce a host of inflammatory and regulatory cytokines, including IL-2, IL-6, IL-10, IL-12p40 and p70, TNF-α and TGF-β. We ensured that in setting up the polarization assays, spleen-derived T cells were exposed to the antigen presenting BMDC along with the cytokines secreted by the BMDC. BMDC infected for 24 h prior to addition of splenocytes were therefore not washed to remove culture medium before 3-day incubation with splenocytes so that the T cells were provided with the first, second and third signals from the APC. In fact, we made sure to carry out gamma irradiation of BMDC in their conditioned medium prior to splenocyte addition.
Other antigens such as purified proteins or synthetic peptides can also be added to BMDC in lieu of live BCG infection and used for T cell polarization assays. - Our protocol therefore limited the cytokines that we could measure by ELISA to assess T cell response, since several cytokines such as IL-2, IL-10 and TNF-α are secreted both by BMDC and T cells. However, cytokine secretion by T cells can be detected by flow cytometry following intracellular cytokine staining.
- Other populations of APC such as DC from spleens or lymph nodes and macrophages obtained from bone marrow cells or tissues such as spleen or peritoneal exudates can also be used as APC in T cell polarization assays. Similarly, T cell lines or clones may be used in lieu of splenocytes. In such cases, the appropriate numbers of cells to be used per well and the ratio of APC to T cells for optimal results should be empirically determined.
- Thymidine, [methyl-3H], 20 Ci/mmol in 70% ethanol, Perkin Elmer, stored at -20 °C. Dilute in complete medium to give 0.5 µCi in 20 µl. Dispense 20 µl per well of the 96 well plate. Avoid adding the radiolabel in very small volumes per well. This will minimize well to well variation.
- BMDC culture. Handle bone marrow cells very gently. Some protocols recommend using an 18 to 20 gauge needle for suspending the clumps of marrow. We have found this reduces viability. The surviving differentiating BMDC are highly phagocytic and engulf these dead cells, leading to their premature and excessive activation.
- GM-CSF. Commercially available native GM-CSF is extremely expensive. Several published papers use recombinant GM-CSF purified from E. coli expressing the protein, which works reasonably well, based on percentage of CD11c-high BMDC by day 7 of culture. However, the BMDC produced with this recombinant GM-CSF can sometimes show undesirably high level of maturity based on surface expression of MHC-II and CD86. We have used culture supernatants of HEK293_T cells stably transfected with a plasmid expressing the mouse GM-CSF and obtained satisfactory preparations of immature BMDC.
- Culture media. Ensure that the IMDM used for culturing BMDC are stringently clean and totally devoid of even traces of endotoxin. We collect water fresh into glass bottles baked at 200 °C, dissolve powdered medium in it and immediately filter through double layer of 0.2 µm membranes inside a biosafety cabinet.
- Fetal bovine serum: Test the batch of serum to ensure that BMDC cultures are immature on day 7. Batches of serum containing high levels of urea, creatinine etc. can cause BMDC activation. Make sure the supplier of the FBS provides data for levels of some 30 odd serum components. Once you find a good batch, order sufficient amounts to last for the duration of these experiments.
- FACS staining for ICC. Check to see if the surface marker-specific antibody conjugate in your panel will recognize its cognate antigen on formaldehyde-fixed cells. If not, you will have to carry out the surface staining first, then fix and permeabilize the cells for intracellular staining. Always titrate all FACS antibodies and use the appropriate quantity to minimize background expansion and spill into other channels. Choice of flours on the FACS antibody should be decided based on the flow cytometer configuration and the bandwidth of filters available on the machine.
- Treatment times with secretion inhibitors brefeldin-A and monensin need to be determined for each antigen used. Potent protein or peptide antigens may require as little as 6 to 12 h stimulation of T cells while our experiments required longer stimulations. While brefeldin-A is not toxic to cells, monensin exposure for prolonged periods can cause cell death. We therefore reduced the monensin concentration from the standard 3 mM to 0.75 mM.
Recipes
- IMDM complete
10% FBS, 2 mM β-mercapto-ethanol, glutamax - Thymidine
[methyl-3H], 20 Ci/mmol in 70% ethanol, stored at -20 °C - Brefeldin A (BFA)
Dissolve 1 mg of BFA in 1 ml of 100% ethanol, and store at -80 °C in aliquots of 50 µl - Monensin
Mol wt: 670 free and 693 for Na salt
Prepare stock of 30 mM (20 mg/ml) in absolute ethanol and store in aliquots of 100 µl at -80 °C - RBC lysis buffer (100 ml)
Ammonium chloride (NH4Cl) 8.99 g
Potassium bicarbonate (KHCO3) 1.0 g
Ethylenediaminetetraacetic acid (EDTA) 37 mg
Dissolve in 80 ml of autoclaved DD water
Adjust the pH to 7.3 using conc. HCl
Make up the volume to 100 ml
Filter using 0.22 µm filter
Do not autoclave
Stored at 2-8 °C - RBC lysis buffer
0.9% NH4Cl in 10 mM Tris (pH 7.5)
Keep sterile at 4 °C
Acknowledgments
This work was funded under the grant BT/PR7240/INF/22/52/2006 from Department of Biotechnology, Ministry of Science and Technology, Government of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Shoba Elangovan and Rajni Nyodu for help with producing the videos. This protocol was adapted from Satchidanandam et al. (2014).
References
- Lutz, M. B. and Rossner, S. (2007). Factors influencing the generation of murine dendritic cells from bone marrow: the special role of fetal calf serum. Immunobiology 212(9-10): 855-862.
- Satchidanandam, V., Kumar, N., Jumani, R. S., Challu, V., Elangovan, S. and Khan, N. A. (2014). The glycosylated Rv1860 protein of Mycobacterium tuberculosis inhibits dendritic cell mediated TH1 and TH17 polarization of T cells and abrogates protective immunity conferred by BCG. PLoS Pathog 10(6): e1004176.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Satchidanandam, V. (2016). Mouse BMDC-dependent T Cell Polarization Assays. Bio-protocol 6(3): e1721. DOI: 10.21769/BioProtoc.1721.
- Satchidanandam, V., Kumar, N., Jumani, R. S., Challu, V., Elangovan, S. and Khan, N. A. (2014). The glycosylated Rv1860 protein of Mycobacterium tuberculosis inhibits dendritic cell mediated TH1 and TH17 polarization of T cells and abrogates protective immunity conferred by BCG. PLoS Pathog 10(6): e1004176.
Category
Immunology > Immune cell function > Lymphocyte
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link