- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cytohistochemical Determination of Calcium Deposition in Plant Cells
Published: Vol 6, Iss 2, Jan 20, 2016 DOI: 10.21769/BioProtoc.1709 Views: 8493
Reviewed by: Marisa RosaRumen IvanovAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Protocol to Measure the Cytoplasmic Calcium in Arabidopsis Guard Cells
Li Li [...] Qun Zhang
May 5, 2015 11454 Views

Cation (Ca2+ and Mn2+) Partitioning Assays with Intact Arabidopsis Chloroplasts
Anna Harms [...] Anja Schneider
Jan 5, 2017 9030 Views

Quantitative Determination of Ca2+-binding to Ca2+-sensor Proteins by Isothermal Titration Calorimetry
Seher Abbas and Karl-Wilhelm Koch
Apr 5, 2020 6317 Views
Abstract
Calcium plays important roles in maintaining plant cellular structure and also acts as a key secondary messenger in intercellular signaling. Thirty years ago, methods of detecting calcium in sub-cellular level had been established (Stockwell and Hanchey, 1982; Borgers et al., 1982) and reviewed extensively (Wick and Heplerm, 1982). We had used the method of testing calcium localization in salt tolerance improved transgenic alfalfa plant (Zhang and Wang, 2015). Here, we describe the protocol of testing calcium deposition by staining with potassium pyroantimonate (PPA) in detail, which was adapted from former reports (Stockwell and Hanchey, 1982; Borgers et al., 1982). The principle of this protocol is that the Ca2+ can react with antimonite and from black granules, which can be observed under a transmission electron microscope. The protocol includes common micromanipulation techniques of plant tissue, observation with a transmission electron microscope and photography.
Keywords: CalciumMaterials and Reagents
- Grids (Sigma-Aldrich, catalog number: G5526 ) for transmission electron microscopy
- 200 μl centrifuge tubes (Eppendorf or other brand)
- 1.5 ml centrifuge tubes (Eppendorf or other brand)
- 50 ml centrifuge tubes (Eppendorf or other brand)
- 0.22 μm Millipore filter unit (Merck Millipore Corporation)
- Plant root tips (cut into ~2 mm in size)
- 25% Glutaraldehyde (Sigma-Aldrich, catalog number: G6257 )
- Disodium hydrogen phosphate dodecahydrate (Na2HPO4) (Sinopharm Chemical Reagent Co., catalog number: 10039-32-4 )
- Potassium pyroantimonate (PPA) (Sigma-Aldrich, catalog number: 60500 )
- 1 M NaOH (Sinopharm Chemical Reagent Co., catalog number: 10019718 )
- Acetone (100%) (Sigma-Aldrich, catalog number: 69508 )
- Ethanol (100%) (Sinopharm Chemical Reagent Co., catalog number: 100092690 )
- Ethylene glycol-O, O′-bis (2-aminoethyl)-1 N, N, N′, N′-tetraacetic acid (EGTA) (Sigma-Aldrich, catalog number: E3889 )
- 1 M HCl (Sinopharm Chemical Reagent Co., catalog number: 10011061 )
- Epon 812 (Sigma-Aldrich, catalog number: 45346 )
- Formvar solution (Sigma-Aldrich, catalog number: 09823 )
Equipment
- Refrigerator (4 °C)
- Incubator
- Vacuum equipment (Vacuum pump connect to a desiccator)
- pH meter
- Transmission electron microscope (Hitachi High-Technologies Europe GmbH, model: H-7500 )
- Ultramicrotome (Leica Microsystems, model: EM UC6 )
- Tweezers (sharp tip)
- Glass beakers
- Glass stirring rods
Procedure
- Preparation of PPA staining solution
- Making 4% PPA solution: Dissolve 8.0 g potassium pyroantimonate (PPA) in the 400 mM Na2HPO4 (pH 7.6) by heating to 90-95 °C and with continuous agitation. Then after, filter the solution through a 0.22 μm Millipore filter, then keep it in 4 °C refrigerator before use (see Notes 1).
- Making PPA staining fluid (50 ml): Mix 25 ml 4% PPA solution with 6 ml 25% Glutaraldehyde, then fill up with 19 ml of sterile H2O (see Notes 2).
- Making 4% PPA solution: Dissolve 8.0 g potassium pyroantimonate (PPA) in the 400 mM Na2HPO4 (pH 7.6) by heating to 90-95 °C and with continuous agitation. Then after, filter the solution through a 0.22 μm Millipore filter, then keep it in 4 °C refrigerator before use (see Notes 1).
- Fixation and rinsing: Collect plant root tips that grown on 1/2 MS medium and rinse with distilled water several times, then cut rip tips with a scalpel in size of about 2 mm and quickly put it in cold PPA staining fluid in a 1.5 ml centrifuge tube. If the plant materials are floating, give a vacuum treatment (0.8 Mpa, 10 min) till the materials sink to the bottom of tube, fixation at 4 °C, overnight. Discard PPA staining fluid, by rinsing with 1% PPA solution for 3 times, each time for 5 min.
- Dehydration: Then after, dehydrate the fixed material in a graded cold acetone serials (30%, 50%, 70%, 90%, 100%, 100%), and 15 min each time.
- Penetration: After dehydration, treat the materials with a serial mixtures of Epon 812: pure acetone (1:3, 1:1 and 3:1) for 12 h each time; and pure Epon 812 mixture (35-40 °C) for 12 h.
- Embed: Add fresh Epon 812 mixture to clean 200 μl Eppendorf tube, transfer root tip carefully and organize the root tip and let it merged in the embedding mixture but not touch to wall of tube with a needle (Figure 1A). Then put the tubes in an incubator, 35 °C for 12 h, then 45 °C for 12 h and 60 °C for 24 h, till the resin is well solidified. Cool down at room temperature for about 1 h.
- Coat nickel grids: Coat nickel grids with formvar can be done by merging the grids in formvar solution for several second then dry on filter paper for about 1 to 2 h; or put a drop of formvar solution directly on the nickel grids on a clean grass slide then let it dry down naturally, it takes about 2 h.
- Sectioning: After trimming the embedded block with a sharp knife (or a block trimmer) properly, cut the block with an ultra-cut microtome to generate 100 nm thick ultrathin sections and mounted onto 200-mesh formvar-coated nickel grids (Figure 1B). Dry under a lamp for about 1 h.
- Microscopy: Check the samples with a transmission electron microscope. Calcium deposition shows black granules or aggregates (Figure 1C-D; Notes 3).
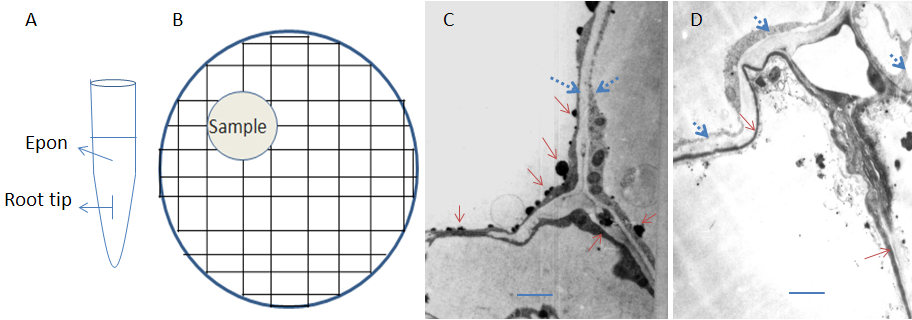
Figure 1. Representative pattern of sampling and calcium deposition in the root-tip cells of alfalfa. A. Illustration of sample in embedding mixture; B. Illustration of sample section on nickel grid; C. Cortical cell walls of rstB transgenic alfalfa showed large aggregates were deposited on remnants of membranes and cytoplasm. D. The epidermal cell walls of wt plant showed weak calcium staining. The deposited granules are smaller. Calcium deposition was indicated with solid red arrows. Lipid bodies (droplets) were indicated with blue dotted arrows. The scale bars represent 2 μm.
Notes
- For plant cells fixation the concentration of Glutaraldehyde could range from 2.5% - 4%. In this protocol 3.0% Glutaraldehyde was used.
- For calcium staining, PPA concentration could range from 0.5% to 2.5% in different protocols.
- To confirm the observed granules are calcium deposits, the section could be treated with 0.1 M EGTA (pH 8.0) at 60 °C for 30 min, and check again. Calcium granules could be removed by EGTA treatment (Stockwell and Hanchey, 1982).
Recipes
- 1 M Na2HPO4 (pH 7.6)
Dissolve 138 g NaH2PO4.H2O in 1 L water and dissolve 142 g of Na2HPO4 in 1 L water to make their 1 M stock solution
During the time, test and modify the pH value with NaOH or HCl to 7.6 - 400 mM Na2HPO4 (pH 7.6)
Mix 338 ml of 1 M Na2HPO4 and 62 ml of 1 M NaH2PO4 and add water to final 1 L - 200 mM Na2HPO4 (pH 7.6)
Dilute 400 mM Na2HPO4 with equal volume of distilled water - 4% PPA solution
In fuming-hood, dissolve 8.0 g potassium pyroantimonate (PPA) in the 400 mM Na2HPO4 (pH 7.6) by heating to 90-95 °C and continuous agitation. - PPA staining fluid (50 ml)
Mix 4% PPA solution 25 ml, 25% Glutaraldehyde 6 ml and 19 ml sterile H2O - 0.1 M EGTA
Add 18.61 g EGTA, 2.0 g NaOH in about 80 ml water with continuous stirring till dissolved completely
Add water to 100 ml
Adjust pH value to 8.0 - 1 M NaOH
Dissolve 4.0 g NaOH in 100 ml water - 1 M HCl
Take 8.36 ml 37% HCl and add water to 100 ml
Acknowledgments
This protocol was an adaption from Stockwell and Hanchey (1982) and Borgers et al. (1982). The work was supported by National Natural Science Foundation of China (31472140). We are grateful to Mrs. H. Hao for her skillful technical assistance in calcium localization tests.
References
- Borgers, M., Thone, F., Verheyen, A. and Ter Keurs, H. E. (1984). Localization of calcium in skeletal and cardiac muscle. Histochem J 16(3): 295-309.
- Stockwell, V. and Hanchey, P. (1982). Cytohistochemical techniques for calcium localization and their application to diseased plants. Plant Physiol 70(1): 244-251.
- Wick, S. M. and Hepler, P. K. (1982). Selective localization of intracellular Ca2+ with potassium antimonate. J Histochem Cytochem 30(11): 1190-1204.
- Zhang, W. J. and Wang, T. (2015). Enhanced salt tolerance of alfalfa (Medicago sativa) by rstB gene transformation. Plant Sci 234: 110-118.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, W. and Wang, T. (2016). Cytohistochemical Determination of Calcium Deposition in Plant Cells. Bio-protocol 6(2): e1709. DOI: 10.21769/BioProtoc.1709.
Category
Plant Science > Plant cell biology > Cell imaging
Plant Science > Plant physiology > Ion analysis
Biochemistry > Other compound > Ion > Calcium
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










