- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Soybean Cyst Nematode, Heterodera glycines, Infection Assay Using Soybean Roots
Published: Vol 6, Iss 2, Jan 20, 2016 DOI: 10.21769/BioProtoc.1707 Views: 11466
Reviewed by: Arsalan DaudiTie LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Rice Ragged Stunt Virus Propagation and Infection on Rice Plants
Chao Zhang [...] Jianguo Wu
Oct 20, 2018 6504 Views

Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2775 Views
Abstract
Soybean cyst nematode (SCN; Heterodera glycines), an obligate parasite of plants, is the most damaging pathogen of soybean, causing $469 to $818 million in soybean yield losses annually in the United States. However, there are no soybean cultivars available that are resistant to all SCN populations. Therefore, much research is being conducted to develop soybean cultivars resistant to SCN (Matthews et al., 2013; Matthews et al., 2014; Youssef et al., 2013). Here we describe the rearing and harvesting of SCN, as well as how SCN can be assayed by determining the Female Index.
Materials and Reagents
- Plastic Pasteur pipettes (Corning, Falcon®, catalog number: 357575 )
- Nylon cloth at 30 micron pore
- 90 mm Whatman circle filter size
- White filter paper (GE Healthcare, catalog number: 10347009 )
- Fleaker filtration unit (Cole Parmer fluid handling and analysis Item) (Cole-Parmer Instrument Compan, catalog number: EW-08917-50 )
- Disposable Petri Dishes (Kord-Valmark Labware Products, catalog number: 2900 )
- Compost soil
- Soybean seeds (William 82 or Essex) (Glycine max) (ARS-USDA)
- Sodium hypochlorite (Commercial Bleach-Clorox contains 8.3% Na-hyperchlorite)
- Sucrose (Baker Analyzed Reagent, catalog number: 4072-1 )
- Table sugar (Commercial sugar)
- Ethyl alcohol (The Warner-Graham Company, catalog number: 64-17-5 )
Note: Currently, it is “Sigma-Aldrich, catalog number: 64-17-5 ”.
- Zinc sulfate heptahydrate (Sigma-Aldrich, catalog number: 91f-0135 )
- Sucrose solution (see Recipes)
- Sodium hypochlorite solution (see Recipes)
- 3 mm Zinc sulfate heptahydrate (ZnSO4) solution (see Recipes)
Equipment
- Greenhouse with a sink to divert drain water to a chlorine holding tank to kill nematodes that go down the drain
- 8-10 inch round Pots (Myers Lawn&Garden)
- Sieves (Sieve sizes: #20 = 841 micron; #60 = 250 micron; #80 = 177 micron; #100 = 149 micron; #250 = 58 micron; #500 = 25 micron) (U.S Standard Sieve Series)
- 1 liter glass cylinder (Pyrex USA)
- Dissecting microscope (Spenser)
- Gyrotory water bath Shaker (LabX, New Brunswick Scientific, model: G76 )
- 1 liter glass beaker (Pyrex)
- Platform Shaker (New Brunswick Scientific)
- 10 ml pipette (Thermo Fisher Scientific, Fisherbrand)
Procedure
Note: All procedures must be in compliance with APHIS requirements to prevent nematodes from escaping the laboratory and greenhouse.
- Culture pots containing soybean plants inoculated with SCN were maintained for three or more months. To collect cysts from stock pots, cut the stem at the soil line of a soybean plant from a stock pot inoculated with SCN for three months. The ball of roots and soil were placed into a two gallon bucket containing water to loosen the root system from the soil ball (Figure 1). How to make a soybean cyst nematodes culture pots was described in Matthews et al. (2013).

Figure 1. Culture pots containing soybean plants inoculated with soybean cyst nematodes
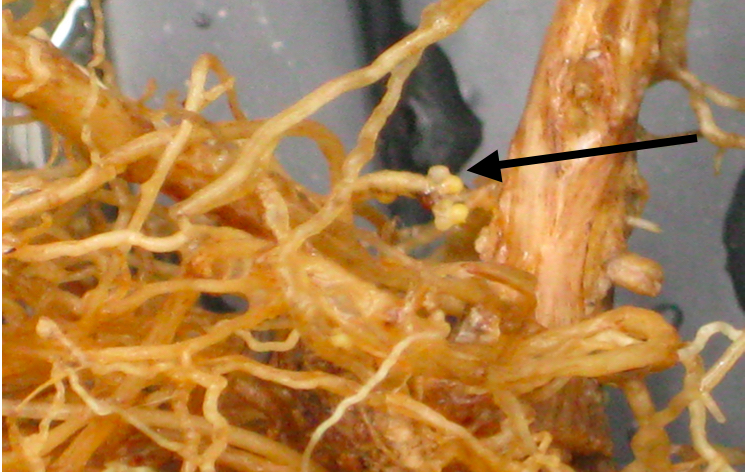
Figure 2. A. Soybean roots containing SCN. (Arrow indicates nematode cyst)
- The roots were placed on nested sieves, #20 on top and #100 on the bottom, as shown in Figure 3. The roots were gently massaged with the fingers and rinsed under running water to collect the cysts and the females on the #100 sieve.

Figure 3. Nested sieves, #20 on top and #100 on the bottom
- The cysts were collected at the edge of the screen by gently washing the inside of the screen with running water. Water containing cysts were gathered at the edge of the screen. The cysts were rapidly poured into a sterile 500 ml plastic beaker.
- Cysts remaining in the soil-water mixture in the bucket were separated from the soil by stirring with a stick. The water was poured into the nested sieves, #20 on top and #100 on the bottom, and the cysts and females were collected on the #100 sieve. This step was repeated for 4 to 5 times.
Note: Autoclave the soil and the roots waste. Treat the bucket with 10% bleach solution to kill any remaining nematodes. We have a valve in the sink drain pipe to divert water containing nematodes into a holding tank for treating the effluent with chlorine.
- The water containing the cysts and females was poured into a 1 L glass cylinder. After 20 to 30 min, the females and cysts sank to the bottom of the cylinder.
- The females and soil were allowed to settle for 5-10 min, and as much water as possible was poured off. The sucrose solution was added to a final volume of 500 ml. A piece of parafilm was placed over the top of the cylinder and turned upside down twice to mix the solution. In 15 min the females and cysts floated to the top of the solution separating them from most of the soil particles and root debris as shown in Figure 4.
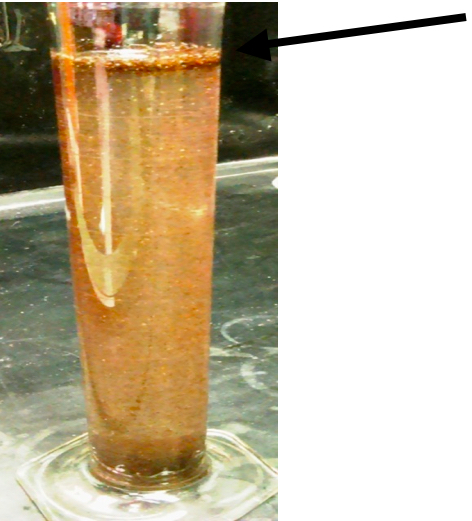
Figure 4. The females and cysts floated to the top of the sucrose solution separating them from most of the soil particles and root debris (the arrow points to the females and cysts)
Note: The solution of sucrose (454 grams/L) was made while waiting for the females to settle, so it is fresh to avoid fungal growth. Table sugar works fine.
- To clean the females and cysts further, they were poured into a 1 L glass beaker. The cylinder was rotated while pouring to recover the females stuck on the walls of the cylinder. The solution from the beaker was poured into a 3 inch diameter #100 sieve and rinsed well to remove the sucrose solution. The sieve was rinsed and contents collected in a 250 ml beaker.
- To clean the females from any remaining root debris, the beaker was rotated to resuspend the females and cysts and allowed to settle a few seconds. The females and cysts were allowed to sink to the bottom of the beaker, because they are heavier than most of the root debris. The root debris was discarded by pouring the water into a glass beaker, leaving the females and cysts in the beaker bottom. This step is repeated several times until most of the root debris was removed.
- To release nematodes and eggs from the cysts, place a 100 sieve containing females on top of an autoclaved 4 cm deep plastic plate (lid of pipet tip box). Add sterile water to just above the level of the sieve. Gently crush the females and cysts with a clean rubber stopper. Occasionally lift the sieve to allow the eggs to fall through to the lid below. Crush the females and cysts for 5 to 10 min. When the water is cloudy, switch sieve to a new autoclaved 4 cm deep plastic plate (lid of pipet tip box) with fresh sterile ddH2O. Crush again. Check the progress of crushing of the females under the microscope. When finished crushing, pour the solution through a 3 inch diameter #250 nested with a #500 sieve. The #250 sieve catches the female and cyst shells. The #500 sieve contains the purified eggs, while the small debris flows through the #500 sieve.

Figure 5. The females and cysts placed on top of an autoclaved 350 ml plate to release the eggs
- Eggs were sterilized by placing the #500 sieve with eggs in an autoclaved 4 cm deep plastic plate (lid of pipet tip box). Bleach solution was added for 1.5 min to the eggs, and the sieve was rotated. The eggs were drained and rinsed with 1 L sterile water. A little sterile water was added to the eggs and the eggs were poured into a fresh sterile 4 cm deep plastic plate (lid of pipet tip box). The volume of the egg and sterile water mixture was brought to 120 ml with sterile water. Then, 1.2 ml sterile 300 mM ZnSO4.7H2O (zinc sulfate heptahydrate) was added to make a final concentration of 3 mM ZnSO4. The ZnSO4 enhances hatching and controls fungal growth. The plate was covered with plastic wrap and placed on a heated shaker at 28 °C at 50-75 rpm for aeration for at least three days. Eggs continued to hatch each day thereafter for over a week. Progress of hatching was monitored using a dissecting microscope. Hatching nematodes are shown in Figure 6.
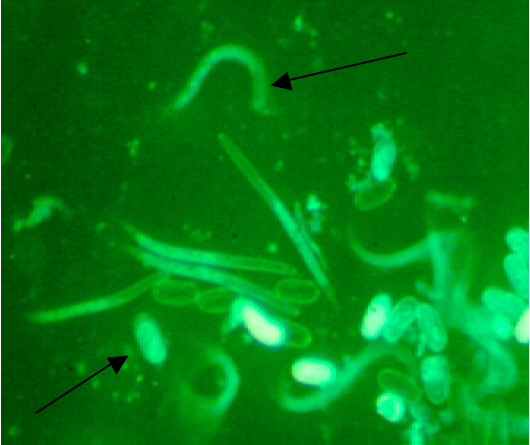
Figure 6. Hached J2 juvenile nematodes and eggs
- J2 stage juveniles were collected by placing a clean nylon cloth of 30 micron pore size into a 1 L glass beaker containing 200 ml reverse osmosis water. The egg/juvenile solution was poured into the nylon cloth and collected. The cloth was dunked like a tea bag into the water for several minutes. This process was repeated with a fresh beaker and water if the juveniles were very concentrated. The volume was increased to no more than 200 ml in a 1 L beaker, and then placed on a shaker at 50-75 rpm to concentrate the juveniles to the center of the beaker. After roughly a half hour, the J2 juveniles were removed with a Pasteur pipette and placed in a 400 ml beaker. Sterile water was added to the desired volume for the experiment and the concentration was checked by pipetting 5 μl of the J2 solution on a drop of water on a microscopic slide to count the nematodes. This was repeated three times and the average was calculated. To get 1,000 J2 per ml, you want 5 J2 per 5 μl solution.
- To inoculate the roots of soybean plants, the plants were grown in sterile sand for 4 weeks. Immediately before inoculation, the plants were watered well then allowed to drain to keep them hydrated. Two holes about 1.5 to 2 cm deep, and 0.5 to 1 cm from the stem were made in the sand on either side of the plant. The solution of nematodes was gently swirled during the inoculation period to keep the nematodes suspended evenly and aerated. A 10 ml pipette was used to measure and dispense the solution of J2 nematodes. To each hole 1 ml of a 1,000 J2/ml solution was added for a total of 2,000 J2/plant. The hole was gently filled with sand. The plants were not watered for two days to give the juveniles a chance to find and migrate into the roots.
- After 30-35 days the females were harvested from each plant, as described in steps 2 and 3. The sand ball containing the soybean roots with nematodes was gently placed in a 1 L plastic beaker. Much of the sand was removed by gently massaging the roots over a beaker. The beaker containing the water and sand were retained for collection of cysts and females. The cell packs or pots were rinsed, and the solution was collected to obtain females that may have adhered to the walls. The solution containing the cysts and females were poured into another and labeled. The roots were gently rubbed under running water to dislodge the cysts and females onto a pair of nested sieves. The #20 sieve allowed the cysts to pass through while keeping out larger debris. The cysts and females were retained on the #100 sieve. The cysts and females were poured into a clean labeled beaker. Cysts and females that were in the sand and in water used to rinse the walls of the pot were collected by passing the water through the nested sieves. More water was added to the beaker and swirled to release cysts and females trapped in the sand. The water was passed through the sieve. This process of rinsing the sand to obtain cysts and females was repeated three times or until the water was clear after swirling. The roots were saved in labeled 100 ml plastic beakers for dry weight measurements.
- The cysts were collected on a lined 90 mm Whatman filter circle using a Fleaker filtration unit (Figure 7). Water was added to wet the filter. The collected nematode solution was added under vacuum. The filter was removed from the unit and a lid was placed on a disposable standard Petri plate. The cysts were counted under a dissecting microscope. The dry weight of each root was recorded in a spreadsheet.

Figure 7. Cysts of SCN collected on lined Whatman filter paper
- Female index calculation. Remove the outliers in the female count data using Grubbs’ test (Grubbs, 1969). A free online version can be found at the GraphPad QuickCalcs Web site (http://graphpad.com/quickcalcs/grubbs1/). Check the normality of the data using the Shapiro-Wilk test (Shapiro and Wilk, 1965). A free, online version implemented by S. Dittamin can be found at http://dittami.gmxhome.de/shapiro/). The female index can be calculated as follows: Female index = (Ng/Nc) X 100, where Ng = mean number of females on roots of experimental plants. Nc = mean number of females present on roots of control plants. Means can be compared using Welch’s unpaired t test for unequal variance (Welch, 1947). A free version is available online at the GraphPad QuickCalcs Web site (http://graphpad.com/quickcalcs/ttest1/). Number of cysts per dry weight of the root can be calculated to standardize the experiment, especially if root size varies greatly.
Recipes
- 1 L of sucrose solution
454 g sucrose
1 L reverse osmosis water
- Sodium hypochlorite solution
Make a 0.4% sodium hypochlorite solution by adding 14.8 ml standard bleach (8.3% sodium hypochlorite) to 285.2 ml sterile water
- 3 mm Zinc sulfate heptahydrate (ZnSO4) solution
1.2 ml sterile 300 mM ZnSO4.7H2O (zinc sulfate heptahydrate) to make a final concentration of 3 mm ZnSO4
Acknowledgments
This protocol is adapted from our previous work, including, Matthews et al. (2013) and Youssef et al. (2013). We thank Peggy MacDonald for technical support. Mention of trade name, proprietary product or vendor does not constitute a guarantee or warranty of the product by the U. S. Department of Agriculture or imply its approval to the exclusion of other products or vendors that also may be suitable. The authors have no conflict of interest.
References
- Grubbs, F. (1969). Procedures for detecting outlying observations in samples. Technometrics 11: 1-21.
- Matthews, B. F., Beard, H., MacDonald, M. H., Kabir, S., Youssef, R. M., Hosseini, P. and Brewer, E. (2013). Engineered resistance and hypersusceptibility through functional metabolic studies of 100 genes in soybean to its major pathogen, the soybean cyst nematode. Planta 237(5): 1337-1357.
- Matthews, B. F., Beard, H., Brewer, E., Kabir, S., MacDonald, M. H. and Youssef, R. M. (2014). Arabidopsis genes, AtNPR1, AtTGA2 and AtPR-5, confer partial resistance to soybean cyst nematode (Heterodera glycines) when overexpressed in transgenic soybean roots. BMC Plant Biol 14: 96.
- Shapiro, S. S. and Wilk, M. B. (1965). Analysis of variance test for normality (complete samples). Biometrika 52: 591-611.
- Youssef, R. M., MacDonald, M. H., Brewer, E. P., Bauchan, G. R., Kim, K. H. and Matthews, B. F. (2013). Ectopic expression of AtPAD4 broadens resistance of soybean to soybean cyst and root-knot nematodes. BMC Plant Biol 13: 67.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Matthews, B. F. and Youssef, R. M. (2016). Soybean Cyst Nematode, Heterodera glycines, Infection Assay Using Soybean Roots. Bio-protocol 6(2): e1707. DOI: 10.21769/BioProtoc.1707.
Category
Plant Science > Plant immunity > Disease bioassay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












