- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Analysis of Murine Lung Tumors by Micro PET-CT Imaging
(*contributed equally to this work) Published: Vol 5, Iss 24, Dec 20, 2015 DOI: 10.21769/BioProtoc.1692 Views: 15431
Reviewed by: HongLok LungAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Monitoring of Sperm-Independent Calcium Oscillations in Immature Oocytes of Mice
Sae Horiike [...] Hidehiko Ogawa
Feb 5, 2026 164 Views

Time-Lapse Into Immunofluorescence Imaging Using a Gridded Dish
Nick Lang [...] Andrew D. Stephens
Feb 20, 2026 48 Views

How to Train Custom Cell Segmentation Models Using Cell-APP
Anish J. Virdi and Ajit P. Joglekar
Feb 20, 2026 40 Views
Abstract
Accurate live tumor imaging in mice is now possible by means of high-resolution positron emission tomography (micro-PET) and X-ray computed tomography (micro-CT). By providing a powerful tool to examine biological samples with complex structure in vivo, this technology generated a significant advance in the cancer research field, particularly regarding the ability to perform longitudinal studies in combination with a therapeutic intervention. Here, we describe methods to optimize visualization of murine lung tumors by micro-PET, micro-CT and combined micro-PET-CT.
Keywords: CancerPart I. Imaging by positron emission tomography (micro-PET)
The imaging of tumors at inner body locations in living animals is more challenging than the imaging of subcutaneous tumors. We have optimized the procedures outlined in the following protocol in order to study lung tumors in genetically modified mice and orthotopic models. Briefly, the mice are anesthetized prior to the administration of the [18F]-Fluorodeoxigluycose (18F-FDG) dose, and kept under anesthesia during the whole period of probe uptake and imaging, ensuring at all times that the mice are warm. The standardization of mouse handling and of anesthesia usage is essential to ensure data reproducibility and comparability.
Materials and Reagents
- 1-cc tuberculin syringes (B. Braun España, model: Omnifix-F )
- 30-G needles (B. Braun España, model: Sterican )
- Heating pads [e.g., Gaymar Mul-T-Pads (Gaymar industries) (http://www.gaymar.com/)]
- Heating pump to maintain temperature of heating pads [e.g., Gaymar TP600 (Gaymar industries) (http://www.gaymar.com/)]
- Genetically modified mouse models bearing endogenous lung tumors (i.e., our own K-RasG12V inducible mouse model, Guerra et al., 2003), lung orthotopic implantation of primary tumors (Ambrogio et al., 2014), or tail-vein injected lung tumor cells (Ambrogio et al., 2014) (Figure 1)

Figure 1. Types of murine lung tumors detectable by PET-CT scan. PET-CT technology can be used to detect lung tumors of different origins: from the left, endogenous lung tumors (i.e., tumors induced by a K-RasG12V resident knock-in allele in a genetically engineered mouse model), tail vein-injected lung cancer cell lines or orthotopically implanted lung tumors (either murine or human lung adenocarcinomas). Scale bar: 200 μM. - Special mouse diets as necessary (Cussó et al., 2014)
- Inhalational anesthesia:
Sevoflurane [e.g., Sevoflo (Abbott Laboratories, catalog number: 05458-02 ) (http://www.abbottanimalhealth.com/veterinary-professionals/products/anesthesia/sevoflo.html)] - Oxygen obtained from an O2 concentrator
- [18F]FDG (0.01 to 0.1 μg/mCi), delivered daily from a local cyclotron (e.g., 40 mCi of [18F]FDG of 95% to 99% radiochemical purity in 1 ml of physiological saline solution buffered at pH 6.0, for ~10 micro-PET scans)
- Physiological saline: 0.9% (w/v) NaCl (B. Braun España)
- Lacryvisc Gel 10 G (3 mg/ml carbomere in benzalconium chloride) (Alcon) (http://www.alcon.com)
Equipment
- O2 concentrator (Eickemeyer Veterinary Equipment, model: Oxymat e3 )
- Infrared heating lamp Philips PAR38 IR 175W E27 (Royal Philips Electronics)
- Sevoflurane/oxygen-based anesthesia system fitted with an induction chamber and inhalation masks for mice McKinley, Type 2 (Everest tecnologia veterinaria)
- Dose calibrator (also known as activimeter) [e.g., VDC-505 dose calibrator (Veenstra Instruments) (http://www.dosecalibrator.com/)]
- micro-PET-CT imaging system [e.g., eXplore Vista PET-CT (GE Healthcare) (Figure 2); Argus PET-CT (SEDECAL) (http://www.sedecal.com/)]

Figure 2. Micro-PET-CT machine (Argus SEDECAL) for mice - Workstation (e.g., Dell PowerEdge) for image acquisition, processing, and analysis meeting the following specifications:
- PE1950 Xeon 5120 1.86 GHz/4 MB 1066 FSB processor
- PE1950 PCIX Riser (2 slots)
- PE1950 Bezel Assembly
- 2 GB FB 667 MHz Memory (2 x 1 GB dual rank DIMMs)
- PE1950 Xeon 5120 1.86 GHz/4 MB 1066 FSB processor
- Alienware Dell Studio XPS Desktop 435 MT PC (for 3DOSEM image reconstruction) meeting the following specifications:
- Processor: Intel Core i7 Quad CPU 940 4 x 2.93 GHz
- Memory: 6144 MB (6 x 1,024) 1067 MHZ DDR3
- Graphics: ATI Radeon HD 3450 256 Mb GDDR2
- Processor: Intel Core i7 Quad CPU 940 4 x 2.93 GHz
Software
- eXplore Vista PET-CT MMWKS software (Desco et al., 2005) or AMIDE software (Loening and Gambhir, 2003) for image acquisition, processing, and analysis
Procedure
- Prepare, sedate and anesthetize mice
- Diet conditions. For the detection of thoracic tumors, mice (as described in Materials and Reagents, Pag. 1) can be fed a specifically formulated commercial high-fat diet (such as, for example, diet D12451 from Research Diets, with 45% of total calories from fat) or, alternatively, sunflower seeds, which are rich in vegetable fats, during the 24 h prior to the analysis. This will decrease glucose uptake by the myocardium, eliminating, to a great extent, the interferences arising from the high uptake of the heart under standard feeding conditions (Cussó et al., 2014).
- Transport mice to the imaging unit the day before the exploration, prepare the diet, and determine their weight.
- Place an infrared lamp above the cage to keep the mice warm prior to the injection.
- Hold the mouse carefully and bring its tail close to the infrared lamp (~5 cm) during 3 to 4 min. Be careful not to expose the whole animal to the lamp, in order to avoid excessive body heating.
- Once the tail veins have dilated, introduce the mice into the anesthesia chamber where a deep anesthesia will be induced by inhalation of 6% sevoflurane in 100% oxygen at a rate of 1.5 L/min.
Note: Alternatively it is possible to use i.p. injection of a mixture containing ketamine (200 mg/kg) and xylazine (10 mg/kg).
- Diet conditions. For the detection of thoracic tumors, mice (as described in Materials and Reagents, Pag. 1) can be fed a specifically formulated commercial high-fat diet (such as, for example, diet D12451 from Research Diets, with 45% of total calories from fat) or, alternatively, sunflower seeds, which are rich in vegetable fats, during the 24 h prior to the analysis. This will decrease glucose uptake by the myocardium, eliminating, to a great extent, the interferences arising from the high uptake of the heart under standard feeding conditions (Cussó et al., 2014).
- Administer the radiolabeled probe
- Transfer anesthetized mice to the injection area and, using a tuberculin shielded syringe and a 30-G needle, inject them with [18F]FDG in the tail (Figure 3) at a dose of 18.5 MBq in a volume of 0.2 ml physiological saline (0.9% NaCl), calibrating the dosage using an activimeter.
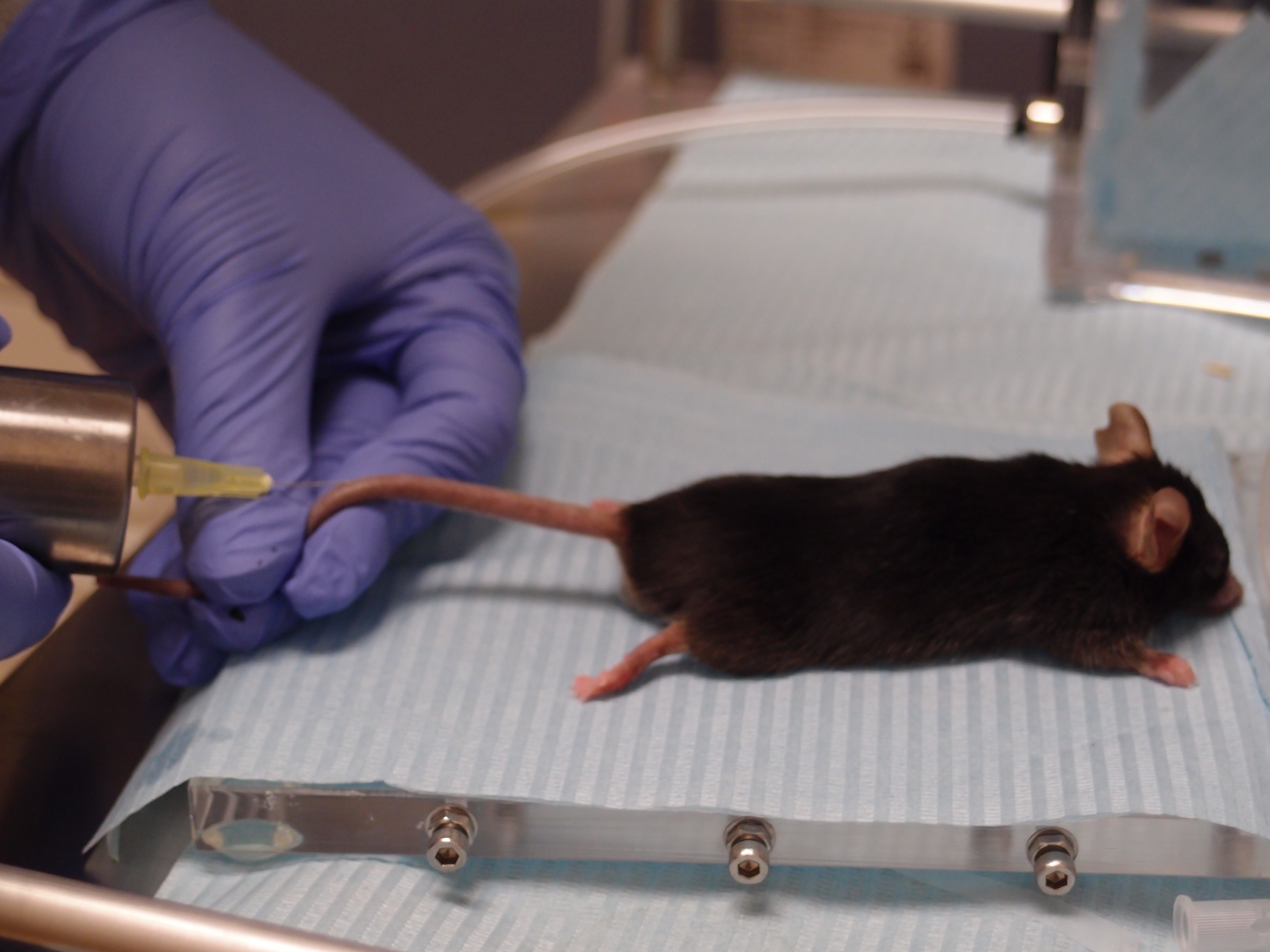
Figure 3. Administration of the probe. Tail vein injection of the radioactive probe into an anesthetized mouse using a shielded syringe. - Once injected, maintain mice under anesthesia providing 3% sevoflurane in 100% oxygen at 0.2 L/min (to avoid muscle uptake due to mice movement) during the [18F]FDG uptake time, which is 30-45 min.
Note: For micro-PET-CT imaging, the micro-CT image acquisition (5 min) is carried out during the [18F]FDG uptake period. Wait for 25-40 min after [18F]FDG administration and then carry out the micro-CT study; the micro-PET study will be performed immediately after the micro-CT one is finished. The anesthesia concentration required during the acquisition of micro-PET-CT imaging (0.2 L/min) is lower than the concentration used for anesthesia induction (1.5 L/min).
- Transfer anesthetized mice to the injection area and, using a tuberculin shielded syringe and a 30-G needle, inject them with [18F]FDG in the tail (Figure 3) at a dose of 18.5 MBq in a volume of 0.2 ml physiological saline (0.9% NaCl), calibrating the dosage using an activimeter.
- Perform image acquisition
- Before placing mice on the exploration table, administer eye lubricant (Lacryvisc Gel 10G) to avoid lesions in the cornea while the mice are anesthetized, since under these conditions the blinking reflex is lost. During image acquisition, mice must be anesthetized with a mask providing 3% sevoflurane in 100% oxygen at 0.2 L/min.
- Set the heating pads on the exploration table of the micro-PET machine at a constant temperature of 37 °C.
- Place mice on the exploration table in a prone position as stretched out as possible to minimize organ superposition.
- Perform image acquisition as described below.
- Perform a blank test. The blank test is an empty acquisition without any radiation. It must be done daily, before any real work is carried out, to assess the correct functioning of the glass detectors of the micro-PET equipment. If the system is working properly, as determined by the blank test, then the user can start the normal operation of the micro-PET. The software program MMWKS VISTA CT (Gleason et al., 2007; Pascau et al., 2012) is launched, the option “PET ACQ” is ticked, and a static study of 5 min duration is selected, without any isotope.
- Select the thoracic area making sure that all lung lobules will be acquired and start the acquisition of the first bed position: the PET scan is acquired as a series of distinct bed motions, called bed positions (usually 1-3 for a standard body scan). At each bed position, the scanner acquires during a set amount of time, then moves approximately 1 cm and begins acquiring the next bed position. All of these individual frames are then knitted together to form the final image, which should take between 10 and 30 min. If we don’t want to see distant metastasis we can avoid the whole body acquisition and reduce the total scan time.
- Divide the acquisition into two parts. Select or create the folder where the study will be saved and create what is known as a scout (a delimitation of the area to be studied), or use a scout previously created (if a micro-CT of the same mouse has already been performed, a scout for this given mouse will already be available). Once the thoracic area has been delimited, select the kind of micro-PET study to be carried out, define the duration of the procedure, the isotope to be used, and the energy window of the isotope of choice (see protocol selection for lung in next point).
- Select the predefined micro-PET acquisition protocol named “static”. This protocol allows for one bed position; it requires previous knowledge of the anatomical region of interest (in this case, thoracic region). This is the protocol of choice for standard lung tumor studies.
- Perform the micro-PET exploration setting the type of isotope to 18F and set the lower energy threshold to 150 KeV. While acquisition is in progress, make sure that the number of coincidences is between 200 and 500. This serves as a quality control for the injection of the radiolabeled compound. The number of coincidences is equivalent to the number of detected photons arising from radioactive disintegrations. If the number of coincidences is not within this range, it will not be possible to perform micro-PET acquisitions. In this last instance, the user should inject another dose of the probe again, so as to have sufficient coincidence events to be detected.
- Perform a blank test. The blank test is an empty acquisition without any radiation. It must be done daily, before any real work is carried out, to assess the correct functioning of the glass detectors of the micro-PET equipment. If the system is working properly, as determined by the blank test, then the user can start the normal operation of the micro-PET. The software program MMWKS VISTA CT (Gleason et al., 2007; Pascau et al., 2012) is launched, the option “PET ACQ” is ticked, and a static study of 5 min duration is selected, without any isotope.
- Once micro-PET acquisition is finished, retrieve mice out of the equipment, disconnect the anesthesia, and take mice to a warm cage where they will wake up on their own.
- Before placing mice on the exploration table, administer eye lubricant (Lacryvisc Gel 10G) to avoid lesions in the cornea while the mice are anesthetized, since under these conditions the blinking reflex is lost. During image acquisition, mice must be anesthetized with a mask providing 3% sevoflurane in 100% oxygen at 0.2 L/min.
- Reconstruct micro-PET image
- Choose an option for image reconstruction. Three options for image reconstruction are available in the eXplore Vista PET-CT equipment: 2D-FBP (filtered back projections), 2D-OSEM (ordered-subsets expectation maximization) and 3D-OSEM. The author’s preference is 3D-OSEM with the number of iterations set to 80 and employing random attenuation and scatter correction. In this manner, we achieve both proper imaging quality and compensation of spatial signal positioning with reasonable computing resources and an acceptable calculation running time.
Note: An independent PC is needed to run 3D-OSEM. The average running time for a 3D-OSEM calculation (80 iterations, random and scatter correction) in a Dell Studio XPS Desktop 435MT PC is <2 min.
- Choose an option for image reconstruction. Three options for image reconstruction are available in the eXplore Vista PET-CT equipment: 2D-FBP (filtered back projections), 2D-OSEM (ordered-subsets expectation maximization) and 3D-OSEM. The author’s preference is 3D-OSEM with the number of iterations set to 80 and employing random attenuation and scatter correction. In this manner, we achieve both proper imaging quality and compensation of spatial signal positioning with reasonable computing resources and an acceptable calculation running time.
- Quantify the data
- Once the total scan volume is reconstructed, inspect the images until a positive [18F]FDG signal is identified in an anatomical position consistent with a lung tumor, and distinct from the normal organs uptake (e.g., heart, urinary bladder, kidneys, and to a lesser extent brown adipose tissue) (Figure 4).
- Manually draw a region of interest (ROI) around the perimeter of the tumor. Subsequently, inspect adjacent sections where the software has automatically drawn the same ROI; if adjustment is needed, it can be done manually.
Note: The 3D-OSEM reconstruction algorithm employs cubic voxels. The software predetermines the size of the reconstructed cubic voxel; in our equipment, its value is of 0.7 x 0.7 x 0.7 mm. Therefore, tumor volumes are built in volume units of cubic voxels. It has to be verified that all the tumor activity has been included. The quantification of [18F]FDG uptake contained in the ROI is performed automatically, expressed in MBq/cc. - From the quantification described under the previous step, calculate the Standardized Uptake Value (SUV).
Note: Two types of SUV measurements can be calculated using the average SUV (SUVave) and the maximum SUV (SUVmax) formulas below:
SUVave = [uptake (MBq)/volume of the ROI (cc)]/[mouse weight (g) x injected [18F]FDG dose (MBq) x calibration factor]
The calibration factor is obtained from acquiring a phantom filled by radiation and is specific for each PET machine. The performance is described in the user manual of each device.
SUVmax = [uptake in the voxel with maximal activity (MBq)/volume of the voxel(cc)]/[mouse weight (g) x injected [18F]FDG dose (MBq) x calibration factor]
SUVave reflects the amount of radioactivity per unit of volume across the entire ROI volume. SUVmax reflects the radioactivity per unit of volume at the voxel, within the ROI, with the maximal value of uptake. In the particular case of mice, due to the great variability in radiolabeled compound distribution, it is advisable to normalize the results of the ROI of interest to a reference/background ROI in normal lung. Data, for example, can be expressed as the ratio of tumor to background (TBR). In Figure 4 a representative image from a lung tumor is depicted, with SUVmax values around 1 to 3 MBq/cc, and SUVmean values around 0.5 to 1 MBq/cc.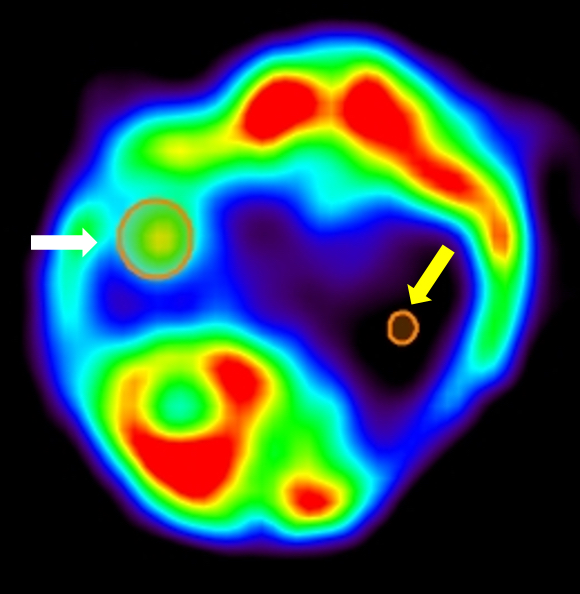
Figure 4. Example of PET quantification. Axial view of a lung tumor PET signal and corresponding ROI of analysis (White arrow). Yellow arrow indicates background ROI placed over the healthy lung.
- Once the total scan volume is reconstructed, inspect the images until a positive [18F]FDG signal is identified in an anatomical position consistent with a lung tumor, and distinct from the normal organs uptake (e.g., heart, urinary bladder, kidneys, and to a lesser extent brown adipose tissue) (Figure 4).
Part II. Imaging by Computed Tomography (micro-CT)
Micro-CT studies are simpler than micro-PET since micro-CT requires little or no preparation. However, mice must be immobilized under anesthetics to avoid artifacts arising from movement. Likewise, in order to prevent anesthesia-induced hypothermia mice must be maintained at constant body temperature by means of a heating pad. Exogenous contrast agents are often used to improve the signal ratio between the tumor and the surrounding healthy tissue, but for the detection of lung tumors contrast is not required.
Materials and Reagents
- Heating pads [e.g., Gaymar Mul-T-Pads (Gaymar industries) (http://www.gaymar.com/)]
- Heating pump to maintain temperature of heating pads [e.g., Gaymar TP600 (Gaymar industries) (http://www.gaymar.com/)]
- Genetically modified mouse models bearing endogenous lung tumors (Guerra et al., 2003), lung orthotopic implantation of primary tumors (Ambrogio et al., 2014), or tail-vein injected lung tumor cells (Ambrogio et al., 2014) (Figure 1)
- Inhalational anesthesia:
Sevoflurane [e.g., Sevoflo (Abbott Laboratories, catalog number: 05458-02 ) (http://www.abbottanimalhealth.com/veterinary-professionals/products/anesthesia/sevoflo.html)] - Oxygen obtained from an O2 concentrator
- Lacryvisc Gel 10 G (3 mg/ml carbomere in benzalconium chloride) (Alcon) (http://www.alcon.com)
Equipment
- O2 concentrator (Eickemeyer Veterinary Equipment, model: Oxymat e3 )
- Infrared heating lamp Philips PAR38 IR 175W E27 (Royal Philips Electronics)
- Sevoflurane/oxygen-based anesthesia system fitted with an induction chamber and inhalation masks for mice McKinley, Type 2 (Everest tecnologia veterinaria)
- Multiparameter Monitor for respiratory gating (Figure 5) [e.g., Vision Vet (RGB) (http://www.medicalexpo.com/prod/rgb-medical-devices/product-69843-493296.html)]
- Infrared heating lamp
- Sevoflurane/oxygen-based anesthesia system fitted with an induction chamber and inhalation masks for mice
- Workstation (e.g., Dell PowerEdge) for image acquisition, processing, and analysis meeting the following specifications:
- PE1950 Xeon 5120 1.86 GHz/4 MB 1066 FSB processor
- PE1950 PCIX Riser (2 slots)
- PE1950 Bezel Assembly
- 2 GB FB 667 MHz Memory (2 x 1 GB dual rank DIMMs)
- PE1950 Xeon 5120 1.86 GHz/4 MB 1066 FSB processor
Software
- eXplore Vista PET-CT MMWKS software (Desco et al., 2005) or AMIDE software (Loening and Gambhir, 2003) for image acquisition, processing, and analysis
Procedure
- Prepare, sedate and anesthetize mice
- Anesthetize mice: Introduce mice into the anesthesia chamber where a deep anesthesia will be induced by inhalation of 6% sevoflurane in 100% oxygen at a rate of 1.5 L/min. and maintain warm; apply some eye lubricant (Lacryvisc Gel 10G) to the cornea for protection as specified in the protocol above before placing mouse inside the scanner chamber. For micro-CT acquisition mice do not require prior preparation (special diet and sedation).
- Before starting the micro-CT, select the thoracic area and properly set the different parameters. The parameters for CT acquisition are usually set within the following ranges of values:
- Intensity of the power supply: from 140 to 1,000 mA.
- Number of shots: From 1 to 32.
Note: The number of shots refers to the number of times that X-rays are emitted from the source. Although a higher number of shots results in a higher signal-to-noise ratio, it also results in larger acquisition times and greater radiation damage inflicted on mice (see step A4 below). - Resolution: standard (200 μm), high (100 μm), or maximum (50 μm).
- Number of projections: From 360◦ to 720◦.
Note: The number of projections refers to the number of rotations of the X-ray beam around the mice, expressed in sexagesimal degrees. Double full rotations (720◦) versus single full rotation (360◦) results in an image of higher signal-to-noise ratio, but at a cost of larger acquisition times and, consequently, greater exposure of the mice to a hazardous energy source and anesthesia. - Number of bed positions: 1 to 3. One bed position for thoracic imaging is enough.
Note: When more than one bed position is required, they superimpose on top of each other, resulting in partial image overlap.
- Intensity of the power supply: from 140 to 1,000 mA.
- Fix mice to the exploration table with adhesive tape even if they are anesthetized, to minimize involuntary movements. Once mice are ready on the exploration table, lower down the protective shielded screen of the Vista-PET-CT and lock in the safety key to perform data acquisition.
- Anesthetize mice: Introduce mice into the anesthesia chamber where a deep anesthesia will be induced by inhalation of 6% sevoflurane in 100% oxygen at a rate of 1.5 L/min. and maintain warm; apply some eye lubricant (Lacryvisc Gel 10G) to the cornea for protection as specified in the protocol above before placing mouse inside the scanner chamber. For micro-CT acquisition mice do not require prior preparation (special diet and sedation).
- Standard micro-CT data acquisition
- The standard acquisition parameters are: Power supply to an intensity of 150 mA and a voltage of 45 kV for a standard resolution of (200 μM), 360◦, and 16 shots for 1 bed position, or 8 shots if performing 2 bed positions. The above acquisition parameters correspond to a radiation dose in the mouse of 0.6 Gy.
- In order to select the acquisition area to observe the scout image generated by the micro-CT machine. This scout gives a projection where the user can see how the subject is placed.
- Select, with the aid of a laser, the beginning and the end of the ROI that the user would like to acquire (e.g., the thoracic region). We can also select a previous scout from a micro-PET study already finished during the same session as long as it corresponds to the same mouse and we have not altered its disposition on the exploration table.
- The standard acquisition parameters are: Power supply to an intensity of 150 mA and a voltage of 45 kV for a standard resolution of (200 μM), 360◦, and 16 shots for 1 bed position, or 8 shots if performing 2 bed positions. The above acquisition parameters correspond to a radiation dose in the mouse of 0.6 Gy.
- Respiratory gating for thoracic CT
- Obtain respiratory signals from an external monitoring device simultaneously with the micro-CT scan (Figure 5; respiratory movement artifacts are important in CT acquisition, especially in the case of thoracic studies). After acquisition, group frames according to the external signal during image processing. This generates two independent scans, corresponding to inhalation and exhalation (grouping into intermediate stages is also possible).
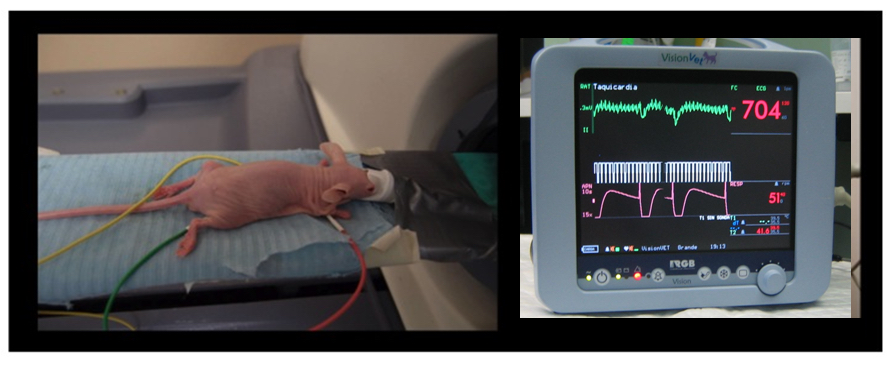
Figure 5. Animal handling and monitoring. Mouse fitted with electrodes being introduced into a PET-CT machine (left panel); monitor of vital constants (VisionVet, RGB http://www.medicalexpo.com/prod/rgb-medical-devices/product-69843-493296.html) registering ECG, temperature, and respiratory frequency (right panel). - Stop the anesthetic delivery once micro-CT acquisition is over, retrieve mice out of the equipment, and place into a warm cage where they will wake up on their own.
- Obtain respiratory signals from an external monitoring device simultaneously with the micro-CT scan (Figure 5; respiratory movement artifacts are important in CT acquisition, especially in the case of thoracic studies). After acquisition, group frames according to the external signal during image processing. This generates two independent scans, corresponding to inhalation and exhalation (grouping into intermediate stages is also possible).
- Micro-CT image reconstruction
- Reconstruct images using a modified version of the cone-beam (CB) algorithm of Feldkamp, Davis, and Kress (FDK, Vaquero et al., 2008) included in the software of the eXplore Vista PET-CT.
Note: FDK is a widely used filtered-back projection algorithm for three-dimensional image reconstruction from cone-beam projections measured with a circular orbit of the X-ray source. Reconstruction time when the algorithm is run on a Dell PowerEdge workstation is <5 sec.
- Reconstruct images using a modified version of the cone-beam (CB) algorithm of Feldkamp, Davis, and Kress (FDK, Vaquero et al., 2008) included in the software of the eXplore Vista PET-CT.
- Quantification using an ROI
- Identify lung tumors by visual inspection of the images.
- Draw manually a region of interest (ROI) around the perimeter of the tumor. Subsequently, inspect adjacent sections where the software draws the same ROI; if adjustment is needed, it can be done manually (Figure 6). The software predetermines the size of the voxels. Our reconstruction software employs cubic voxels of 50 x 50 x 50 μm to define the ROI. The volume of the selected ROI, expressed in cubic centimeters, is automatically calculated by the system.
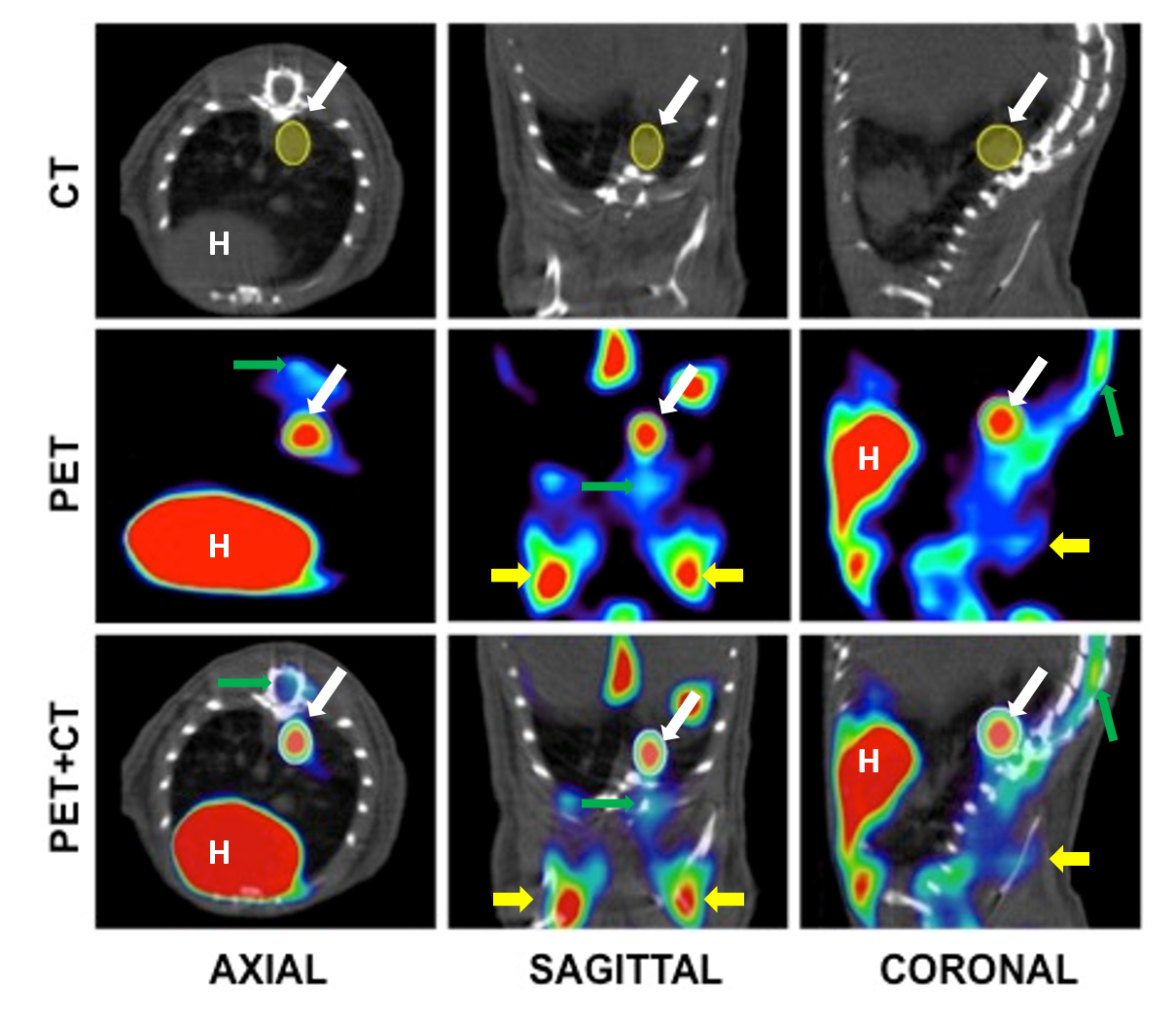
Figure 6. Example of ROI selection in a CT, PET and fusion images. The example shows (from left to right) axial, sagittal and coronal sections of the chest cavity. The ROI, visible in all three sections, is shown in solid yellow. H: Heart. Tumor is indicated with a white arrow. Green arrow points bone marrow and yellow arrows mark brown adipose tissue.
- Identify lung tumors by visual inspection of the images.
Part III. Imaging by multimodality (micro-PET-CT)
The immobilization of mice during the exploration is essential for image co-registration in multimodality imaging studies. If positions during micro-PET and micro-CT studies are different, the matching (co-registration) of the images obtained by micro-PET and micro-CT is not possible.
Materials and Reagents
- 1-cc tuberculin syringes (B. Braun España, model: Omnifix-F )
- 30-G needles (B. Braun España, model: Sterican )
- Heating pads [e.g., Gaymar Mul-T-Pads (Gaymar industries) (http://www.gaymar.com/)]
- Heating pump to maintain temperature of heating pads [e.g., Gaymar TP600 (Gaymar industries) (http://www.gaymar.com/)]
- Genetically modified mouse models bearing endogenous lung tumors (Guerra et al., 2003), lung orthotopic implantation of primary tumors (Ambrogio et al., 2014), or tail-vein injected lung tumor cells (Ambrogio et al., 2014) (Figure 1)
- Special mouse diets as necessary (Cussó et al., 2014)
- Inhalational anesthesia:
Sevoflurane [e.g., Sevoflo (Abbott Laboratories, catalog number: 05458-02) (http://www.abbottanimalhealth.com/veterinary-professionals/products/anesthesia/sevoflo.html)] - Oxygen obtained from an O2 concentrator
- [18F]FDG (0.01 to 0.1 μg/mCi), delivered daily from a local cyclotron (e.g., 40 mCi of [18F]FDG of 95% to 99% radiochemical purity in 1 ml of physiological saline solution buffered at pH 6.0, for ~10 micro-PET scans)
- Physiological saline: 0.9% (w/v) NaCl (B. Braun España)
- Lacryvisc Gel 10 G (3 mg/ml carbomere in benzalconium chloride) (Alcon) (http://www.alcon.com)
Equipment
- O2 concentrator (Eickemeyer Veterinary Equipment, model: Oxymat e3 )
- Infrared heating lamp Philips PAR38 IR 175W E27 (Royal Philips Electronics)
- Sevoflurane/oxygen-based anesthesia system fitted with an induction chamber and inhalation masks for mice McKinley, Type 2 (Everest tecnologia veterinaria)
- Dose calibrator (also known as activimeter) [e.g., VDC-505 dose calibrator (Veenstra Instruments) (http://www.dosecalibrator.com/)]
- micro-PET-CT imaging system [e.g., eXplore Vista PET-CT (GE Healthcare, Figure 2); Argus PET-CT (SEDECAL) (http://www.sedecal.com/)]
- eXplore Vista PET-CT MMWKS software (Desco et al., 2005) or AMIDE software (Loening and Gambhir, 2003) for image acquisition, processing, and analysis
- Workstation (e.g., Dell PowerEdge) for image acquisition, processing, and analysis meeting the following specifications:
- PE1950 Xeon 5120 1.86 GHz/4 MB 1066 FSB processor
- PE1950 PCIX Riser (2 slots)
- PE1950 Bezel Assembly
- 2 GB FB 667 MHz Memory (2 x 1 GB dual rank DIMMs)
- PE1950 Xeon 5120 1.86 GHz/4 MB 1066 FSB processor
- Alienware Dell Studio XPS Desktop 435 MT PC (for 3DOSEM image reconstruction) meeting the following specifications:
- Processor: Intel Core i7 Quad CPU 940 4 x 2.93 GHz
- Memory: 6144 MB (6 x 1,024) 1067 MHZ DDR3
- Graphics: ATI Radeon HD 3450 256 Mb GDDR2
- Processor: Intel Core i7 Quad CPU 940 4 x 2.93 GHz
Procedure
- Prepare mice
- Prepare mice prior to the multimodality exploration as described for the micro-PET exploration in the corresponding protocol, steps 1 to 6.
Note: Multimodality exploration requires extreme attention to the care of mice as well as constant monitoring of the temperature, since the study times are longer than in CT or PET alone.
- Prepare mice prior to the multimodality exploration as described for the micro-PET exploration in the corresponding protocol, steps 1 to 6.
- Acquire micro-CT image
- Perform the acquisition of the micro-CT image during the [18F]FDG uptake period (lasting 45 min). See micro-PET protocol, step 8, and micro-CT protocol.
- Acquire PET image
- Perform micro-PET image acquisition following the same procedure as described in the corresponding protocol, steps 7 to 13.
- Reconstruct images
- Reconstruct both images separately and use the MMWKS software to obtain the co-register (Figures 6-7).
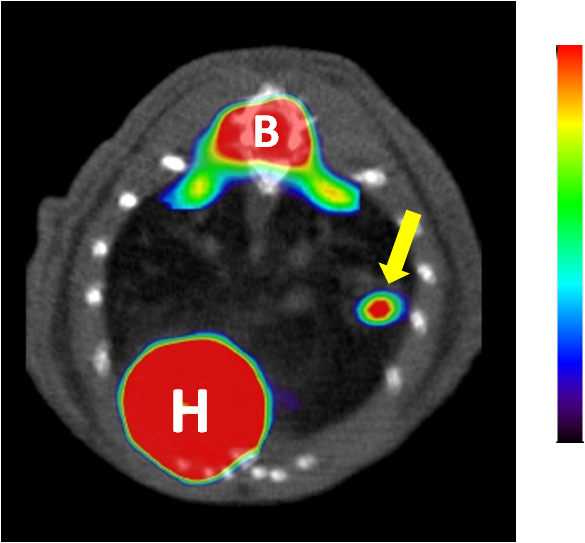
Figure 7. PET-CT imaging of a lung tumor. Representative axial projection of FDG-PET scans showing tumor FDG-PET signal (indicated by an arrow). H: heart, B: Bone marrow. Tumor is indicated with a yellow arrow.
- Reconstruct both images separately and use the MMWKS software to obtain the co-register (Figures 6-7).
- Quantify images
- Once lung tumors have been identified, quantify the micro-CT image and the micro-PET image separately, as described in the protocols above.
- Rendering of volumes
- Generate 3-D renderings of the data to obtain information on lung tumor location and volume in a more graphical way (Figure 8).
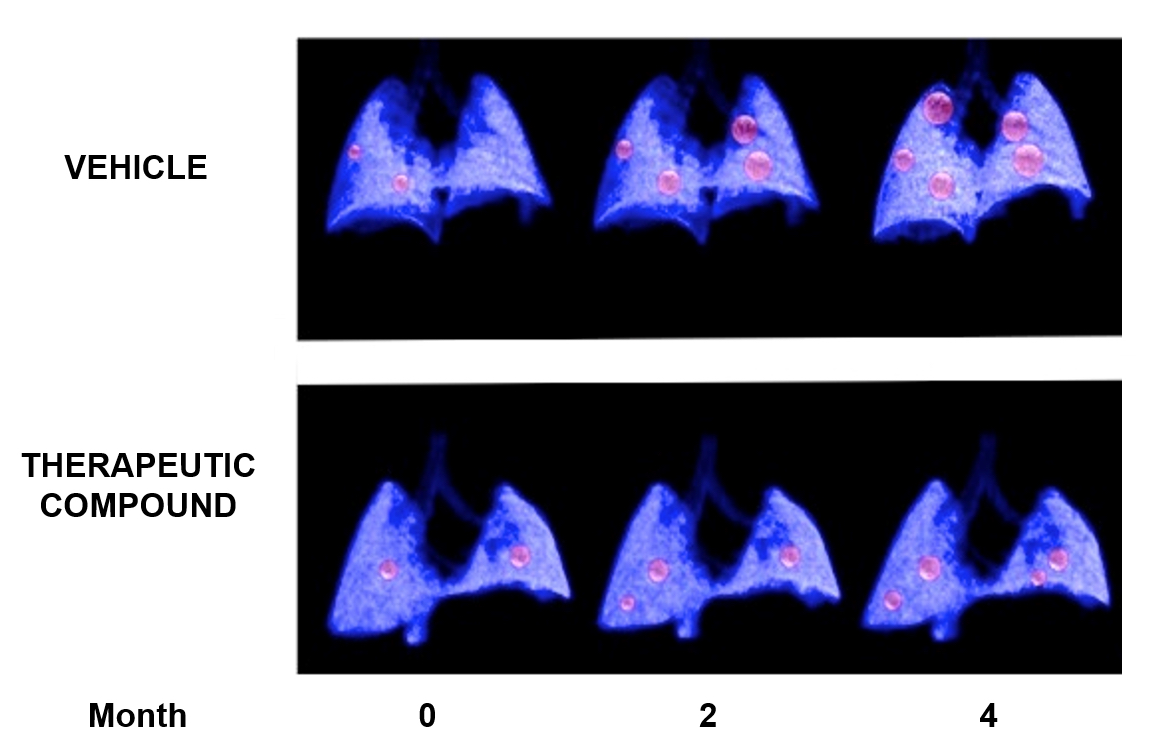
Figure 8. Example of a longitudinal study of lung cancer. 3-D lung renderings of PET-CT studies at the indicated time-points of two individual mice carrying genetically induced lung tumors (in red), either treated with vehicle (upper panel) or with a therapeutic compound (lower panel).
- Generate 3-D renderings of the data to obtain information on lung tumor location and volume in a more graphical way (Figure 8).
Acknowledgments
This protocol has been adapted from the previously published study “Imaging Cancer in Mice by PET, CT, and Combined PET-CT” (Mulero et al., 2011). We are grateful to the Molecular Imaging Core Unit for the expertise and key contributions to the optimization of the protocols described here. This work was supported by grants from the CDTI (Spanish center of industrial and technological development Spanish Ministry of Science), AMIT Project “Advanced Molecular Imaging technologies” (5710001425) to FM. CA is the recipient of a postdoctoral fellowship from the Spanish Association Against Cancer (AECC).
References
- Ambrogio, C., Carmona, F. J., Vidal, A., Falcone, M., Nieto, P., Romero, O. A., Puertas, S., Vizoso, M., Nadal, E., Poggio, T., Sanchez-Cespedes, M., Esteller, M., Mulero, F., Voena, C., Chiarle, R., Barbacid, M., Santamaria, D. and Villanueva, A. (2014). Modeling lung cancer evolution and preclinical response by orthotopic mouse allografts. Cancer Res 74(21): 5978-5988.
- Cusso, L., Vaquero, J. J., Bacharach, S. and Desco, M. (2014). Comparison of methods to reduce myocardial 18F-FDG uptake in mice: calcium channel blockers versus high-fat diets. PLoS One 9(9): e107999.
- Desco, M., Penedo, M., Gispert, J. D., Vaquero, J. J., Reig, S. and Garcia-Barreno, P. (2005). ROC evaluation of statistical wavelet-based analysis of brain activation in [15O]-H2O PET scans. Neuroimage 24(3): 763-770.
- Gleason, S. S., Austin, D. W., Beach, R. S., Nutt, R., Paulus, M. J., and Yan, S. (2007). A new highly versatile multimodality small animal imaging platform. In: IEEE nuclear science symposium conference record. pp 2447-2449.
- Guerra, C., Mijimolle, N., Dhawahir, A., Dubus, P., Barradas, M., Serrano, M., Campuzano, V. and Barbacid, M. (2003). Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell 4(2): 111-120.
- Loening, A. M. and Gambhir, S. S. (2003). AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2(3): 131-137.
- Mulero, F., Donate, L. E. and Serrano, M. (2011). Imaging Cancer in Mice by PET, CT, and Combined PET-CT. Curr Protoc Mouse Biol 1(1): 85-103.
- Pascau, J., Vaquero, J. J., Chamorro-Servent, J., Rodriguez-Ruano, A. and Desco, M. (2012). A method for small-animal PET/CT alignment calibration. Phys Med Biol 57(12): N199-207.
- Vaquero, J. J., Redondo, S., Lage, E., Abella, M., Sisniega, A., Tapias, G., Soto Montenegro, M. L., and Deseo, M. (2008). Assessment of a new high-performance a small-animal X-ray tomograph. In: IEEE transactions on nuclear science. pp 898-905.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ambrogio, C., Cámara, J. A., Nieto, P., Santamaría, D. and Mulero, F. (2015). Analysis of Murine Lung Tumors by Micro PET-CT Imaging. Bio-protocol 5(24): e1692. DOI: 10.21769/BioProtoc.1692.
Category
Cancer Biology > General technique > Tumor formation
Cell Biology > Cell imaging > Live-cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link