- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
[14C] Linoleic Acid Uptake and Fractionation Assay in Vibrio cholerae
Published: Vol 5, Iss 24, Dec 20, 2015 DOI: 10.21769/BioProtoc.1682 Views: 7246
Reviewed by: Valentine V TrotterElizabeth LibbyAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Extraction of Bacterial Membrane Vesicle and Phage Complex by Density Gradient Ultracentrifugation
Shangru Li [...] Tianyuan Jia
Aug 20, 2024 2054 Views

Mycobacterium smegmatis Ribosome Purification, Co-sedimentation, and Subunit Association Assay
Aneek Banerjee [...] Jayati Sengupta
May 20, 2025 1579 Views

Preparation and Negative Staining for Visualization of Cyanoglobule Lipid Droplets Using Transmission Electron Microscopy
Febri A. Susanto [...] Peter K. Lundquist
Dec 5, 2025 1311 Views
Abstract
The gram-negative curved bacillus Vibrio cholerae (V. cholerae) causes the severe diarrheal illness cholera. The work presented here is to assess whether unsaturated fatty acids (UFAs), such as linoleic acid, have the potential to directly affect proteins involved in DNA binding because they are able to enter the cell. In this protocol, we show how to measure linoleic acid entering V. cholerae when added exogenously and determine whether it is able to enter the cytoplasm. This protocol will quantify how much linoleic acid is able to enter the cell and then identify the amount of linoleic acid that stays in the membrane or ultimately enters the cytoplasm.
Keywords: Vibrio choleraeMaterials and Reagents
- Scintillation vials (Thermo Fisher Scientific, catalog number: 03-337-20 )
- Autoclaved 1.7 ml microcentrifuge tubes (BioExpress, catalog number: C-3262-1 )
- Test Tubes (Thermo Fisher Scientific, catalog number: 14-955E )
- 15 ml conical screw cap tubes (BioExpress, catalog number: C-3394-2 )
- Vibrio cholerae classical biotype strain O395
- 14C linoleic acid (PerkinElmer, catalog number: NEC501050UC )
- Scintillation cocktail (Thermo Fisher Scientific, catalog number: SX18-4 )
- Tryptone (Thermo Fisher Scientific, catalog number: B211705 )
- Yeast Extract (Thermo Fisher Scientific, catalog number: B288620 )
- Sodium chloride (NaCl) (Thermo Fisher Scientific, catalog number: BP358-212 )
- Potassium chloride (KCl) (Thermo Fisher Scientific, catalog number: BP366-500 )
- Sodium phosphate (Na2HPO4) (Thermo Fisher Scientific, catalog number: BP332-500 )
- Potassium phosphate (KH2PO4) (Thermo Fisher Scientific, catalog number: BP362-500 )
- Tris-Base (Thermo Fisher Scientific, catalog number: BP152-500 )
- 95% ethanol (Decon Labs, catalog number: 2805HC )
- Dry ice
- LB-Lennox (pH 6.5) (see Recipes)
- 10x PBS (see Recipes)
- 20 mM Tris-Base (pH 8.5) (see Recipes)
- 500 mM NaCl (see Recipes)
Equipment
- Shaker-capable of shaking at 200 rpm at 37 °C (VWR International, New Brunswick Scientific, model: Excella E25 )
- Water bath shaker-capable of shaking at 30 °C at 200 rpm (VWR International, New Brunswick Scientific, model: Classic C76 )
- LS6000IC liquid scintillation counting system (Beckman Coulter)
- Timer
- Autoclave
- Biomate 3S Spectrophotometer-capable of reading at OD600 (Thermo Fisher Scientific)
- Semimicro Cuvettes (Thermo Fisher Scientific, catalog number: 14-955-127 )
- Table top Centrifuge-capable of spinning at 15,000 rpm at 4 °C (Eppendorf, catalog number: 5424 )
- Micropipettes (1,000 μl, 200 μl, 20 μl)
- 250 ml Erlenmeyer flask
Procedure
- [14C] linoleic acid uptake
- Use one colony of V. cholerae classical biotype strain O395 to start an overnight culture in a test tube containing 5 ml standard LB at 37 °C and 200 rpm shaking.
- After overnight growth, subculture the bacteria 250 μl in 10 ml LB pH 6.5 (1:40 ratio) in an Erlenmeyer flask, and grow for 2 h in a water bath shaker at 30 °C.
- At 2 h, record the OD600 of the culture. Expect an OD600 of between 0.2 and 0.3.
- Transfer the culture to a 15 ml falcon tube and add 0.1 μCi (1 μl) of 14C-radiolabeled linoleic acid for each milliliter of the subculture (final concentration of about 3.2 mM linoleic acid). This tube can stay on the bench top for the duration of the experiment or, if desired, put at 37 °C without agitation between aliquots.
- Upon addition of the radiolabeled linoleic acid, extract 1 ml of the culture and immediately centrifuge in a 1.5 ml microcentrifuge tube at 15,000 rpm for 3 min at room temperature. This is t = 0. In order to compare the counts per minute (CPM) of 14C in the supernatant and the cell pellet, transfer the supernatant in a 1.5 ml tube and wash the cell pellet 3 times with 1 ml of 1x PBS and centrifuge each for 3 min.
- Resuspend the cell pellet in 100 μl of 1x PBS and add to 5 ml of scintillation cocktail per scintillation vial. In order to compare the amount of 14C-radiolabeled linoleic acid not taken up by V. cholerae, add 100 μl of the supernatant fraction to another vial with 5 ml scintillation cocktail.
- The same procedure can be repeated for other aliquots of the subculture at times 5, 15, and 30 min or whatever time points are desired. All scintillation vials can be stored at room temperature and read together after the last time point.
- After uptake, counts per minute are measured for each time point using a scintillation counting system.
- Data are then analyzed using the cpm determined from the cell pellet fraction and an equation of best fit determined. To determine how much 14C-radiolabeled linoleic acid is not taken up as a check of accuracy, use cpm determined from the supernatant fraction and multiply by 10 as only 100 μl of the 1 ml was used. The amount of 14C-radiolabeled linoleic acid in the cell pellet is graphed below for each time point:
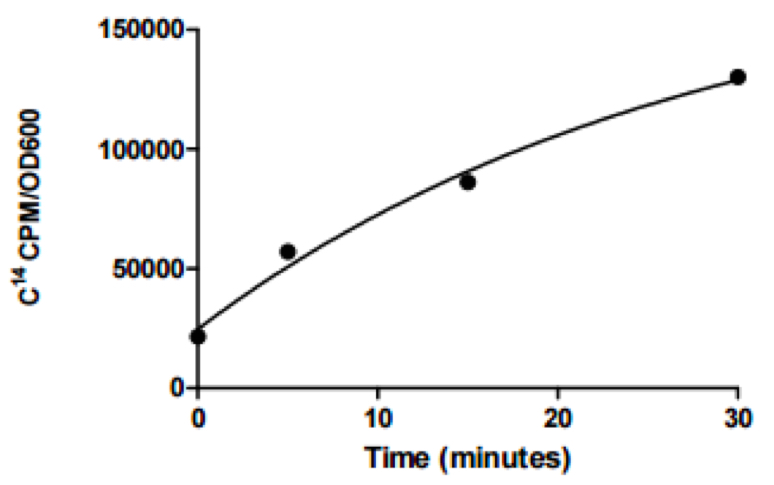
Figure 1. 14C-radiolabeled linoleic acid uptake by V. cholerae. The equation of best fit is y = 24622 + 156786*(1 - e-0.036x) with an R2 value of 0.989.
- Use one colony of V. cholerae classical biotype strain O395 to start an overnight culture in a test tube containing 5 ml standard LB at 37 °C and 200 rpm shaking.
- Fractionation of V. cholerae to determine localization of [14C] linoleic acid
- After overnight growth at 37 °C as described in step A1, subculture V. cholerae O395 classical biotype 250 μl in 10 ml LB (pH 6.5) (1:40 ratio) and grow in a water bath shaker at 30 °C for 2 h.
- At 2 h, add 0.1 μCi of 14C-radiolabeled linoleic acid to 1 ml of the subculture and incubate at room temperature for 1 h without agitation. This time is chosen to ensure the bacteria are still in log-phase growth.
- Harvest the bacteria by centrifugation (15,000 rpm) at room temperature for 3 min and wash the pellet three times with 1 ml 1x PBS at 15,000 rpm for 3 min each time.
- Resuspend the bacteria in a 500 μl solution of 20 mM Tris-Base (pH 8.5) and 500 mM NaCl. Freeze the suspension in an ethanol (at least 95%) and dry ice bath for 2 minutes ensuring the tube is fully submerged, and then put at 37 °C until thawed. Repeat this freeze-thaw process for a total of three freeze-thaw cycles to ensure complete fractionation.
- Fractionate the bacteria by centrifugation for 10 min at 15,000 x g at 4 °C to separate the membrane and cytoplasm. The cytoplasm is taken as the supernatant and the pellet (cell envelope fraction) is washed 3 times in 500 μl of 1x PBS by centrifuging for 3 min at 15,000 x g. Expect a very small pellet at the bottom of the tube. The amount of 14C-linoleic acid in each fraction is determined by adding the entire fraction to 5 ml scintillation cocktail, followed by measurement of CPM in a scintillation counter.
- Data are then analyzed as percent 14C-linoleic acid in each fraction. A representative graph is shown below:
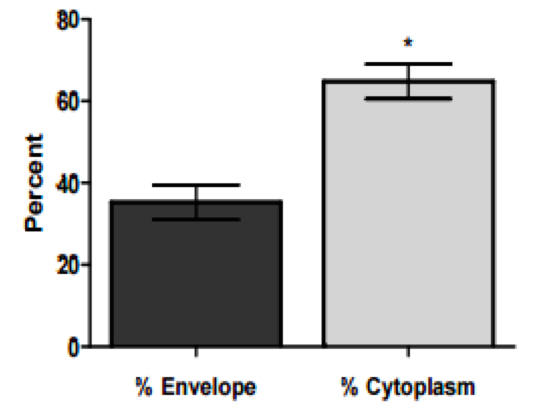
Figure 2. Percentages of 14C-radiolabeled linoleic acid in cytoplasm and envelope fractions
- After overnight growth at 37 °C as described in step A1, subculture V. cholerae O395 classical biotype 250 μl in 10 ml LB (pH 6.5) (1:40 ratio) and grow in a water bath shaker at 30 °C for 2 h.
Recipes
- LB-Lennox (500 ml) (pH 6.5)
5 g tryptone
2.5 g NaCl
2.5 g yeast extract
Add about 400 ml of water to dissolve and mix the components
pH to 6.5 with HCl and bring up to 500 ml with water
Autoclave before use - 10x PBS (1 L)
80 g NaCl
2 g KCl
14.4 g Na2HPO4
2.4 g KH2PO4
To use, dilute 1:10 (such as 10 ml brought up to 100 ml with autoclaved water) - 20 mM Tris-base (pH 8.5) (100 ml)
0.24 g Tris-base
pH to 8.5 with HCl and bring up to 100 ml with water
Autoclave before use - 500 mM NaCl (100 ml)
2.92 g NaCl
Acknowledgments
This work was supported by P. H. S. grants K22AI071011 and R56AI093622 and Bill and Melinda Gates Foundation grant OPP1068124 (to J. H. W.).
This work is modified from previous work done in our laboratory (Thomson and Withey, 2014).
References
- Plecha, S. C. and Withey, J. H. (2015). Mechanism for inhibition of Vibrio cholerae ToxT activity by the unsaturated fatty acid components of bile. J Bacteriol 197(10): 1716-1725.
- Thomson, J. J. and Withey, J. H. (2014). Bicarbonate increases binding affinity of Vibrio cholerae ToxT to virulence gene promoters. J Bacteriol 196(22): 3872-3880.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Plecha, S. C. and Withey, J. H. (2015). [14C] Linoleic Acid Uptake and Fractionation Assay in Vibrio cholerae. Bio-protocol 5(24): e1682. DOI: 10.21769/BioProtoc.1682.
Category
Microbiology > Microbial biochemistry > Lipid
Microbiology > Microbial cell biology > Organelle isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









