- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Lymphocytes from Murine Visceral Adipose Tissue
Published: Vol 5, Iss 23, Dec 5, 2015 DOI: 10.21769/BioProtoc.1669 Views: 17985
Reviewed by: Shanie Saghafian-HedengrenAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Generation of T cells from Human and Nonhuman Primate Pluripotent Stem Cells
Akhilesh Kumar [...] Igor I. Slukvin
Jul 5, 2020 7286 Views

Expansion and Polarization of Human γδT17 Cells in vitro from Peripheral Blood Mononuclear Cells
Xu Chen [...] Jun Yan
Jan 5, 2024 1951 Views

Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
Jun 5, 2025 2620 Views
Abstract
Several studies have shown that the detrimental influence of abdominal obesity on metabolic processes is mediated by the intra-abdominal fat depot. Visceral adipose tissue has been shown to be an independent risk factor for coronary heart disease, hypertension, impaired glucose tolerance and Diabetes Mellitus Type 2 (DM2). Diet-induced obesity in mice, primarily of the C57BL/6J strain, is a commonly used method to study the development of insulin resistance as a model for DM2. The white or visceral adipose tissue (here referred to as VAT), especially the fat around the gonads, is a commonly used organ of study in this model, as it accumulates large numbers of lymphocytes in response to diet-induced obesity. The protocol below describes the isolation of lymphocytes from the stromal vascular fraction (SVF) from VAT.
Materials and Reagents
- 50 ml centrifuge tubes
- 70 µm cell strainer (BD Biosciences, Falcon®, catalog number: 352350 )
- Male mouse (e.g., C57BL/6J) 8-20 weeks old
Note: Generally, male mice are more severely affected by type 2 diabetes than female mice, and they are used exclusively in diet-induced diabetes studies (www.jax.org). - Collagenase from Clostridium histolyticum type IV (Sigma-Aldrich)
- Fetal Bovine Serum (FBS) (Pan biotech GmbH)
- Trypan blue
- RPMI 1640 (with L-glutamine; 25 mM Hepes; 2.2 g/L NaHCO3) (Pan Biotech GmbH)
- MilliQ water
- 0.83% NH4Cl
- 0.168% Na2CO3
- 1 mM EDTA (pH 7.3)
- 1x PBS (pH 7.3)
- 0.2% BSA
- 3% RPMI 1640 (see Recipes)
- 3% RPMI + 1 mg/ml Collagenase D (or IV) (see Recipes)
- Erylysis buffer (see Recipes)
- FACS wash buffer (see Recipes)
Equipment
- Soft wood tablet and pins
- Disinfectant
- Small thin surgical scissors and tweezers
- Thermostatic shaker
- Vortex
- Vacuum pump
- Centrifuge
Procedure
- Euthanize the mouse by O2/CO2 (70%/30%) intoxication, followed by CO2 asphyxiation. Of note, all experiments using mice were approved beforehand by your Institutional Animal Care and Use Committee and were in accordance with national and international guidelines.
- Gently lay down the mouse on its back, on a soft wood surface, stretch the limbs and fix the four paws with pins (Figure 1).

Figure 1. Fixation. Fix the animal on its back to facilitate VAT removal. - Clean the abdomen with disinfectant.
- Use a scissor to make a midline incision (Figure 2) and use straight tweezers to retract the skin. Open the muscular wall with another cutting tool. Steps 4-6 are also illustrated in Video 1.Video 1. Excision of VAT
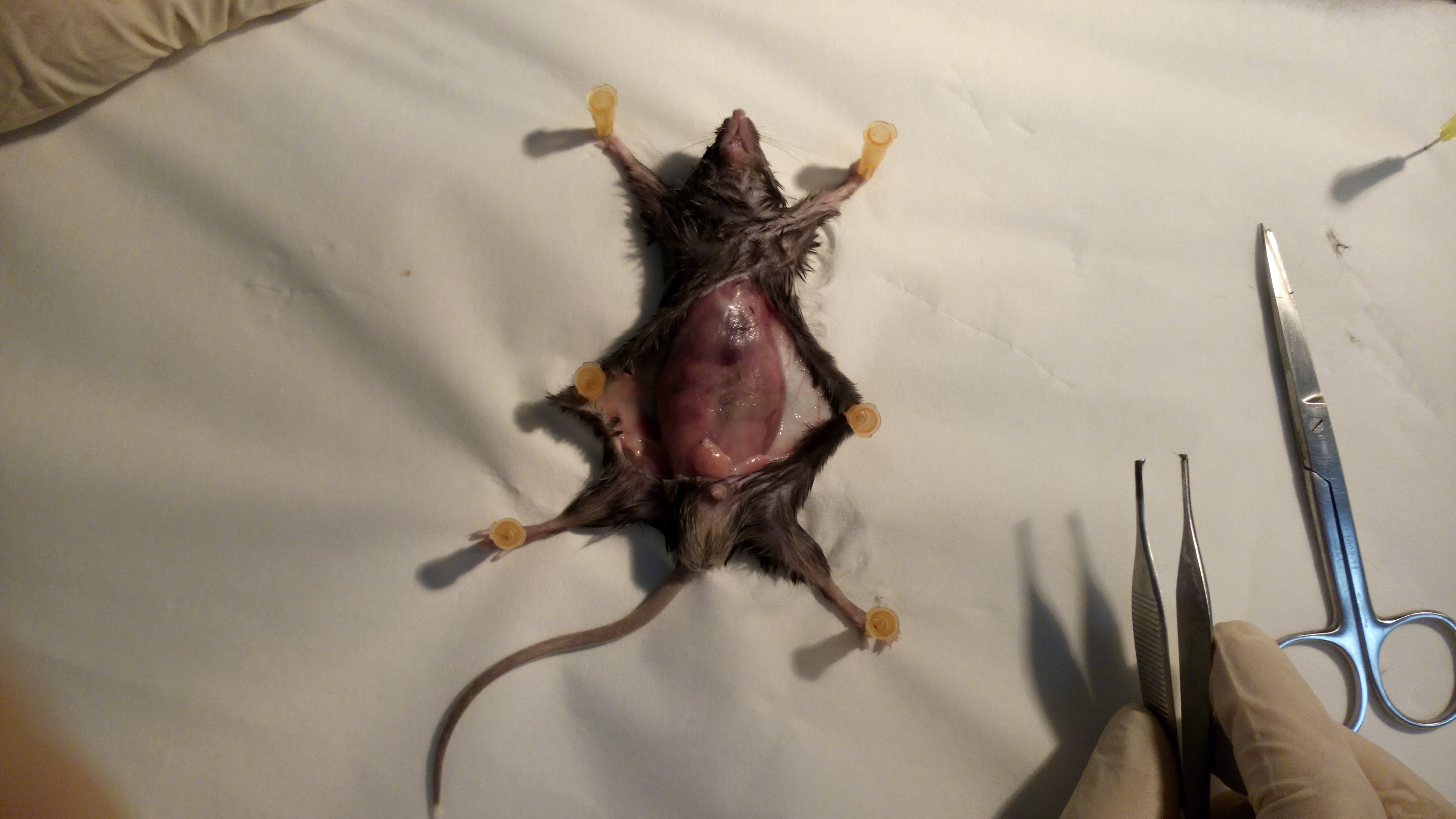
Figure 2. Opening of the skin. First remove the skin to prevent damage to the internal organ by cutting too deep. - The peritoneum contains several adipose tissue reservoirs. Draw out the white or visceral (perigonadal) adipose tissue (VAT). The perigonadal fat expands most vigorously upon high-fat feeding (Figure 3).
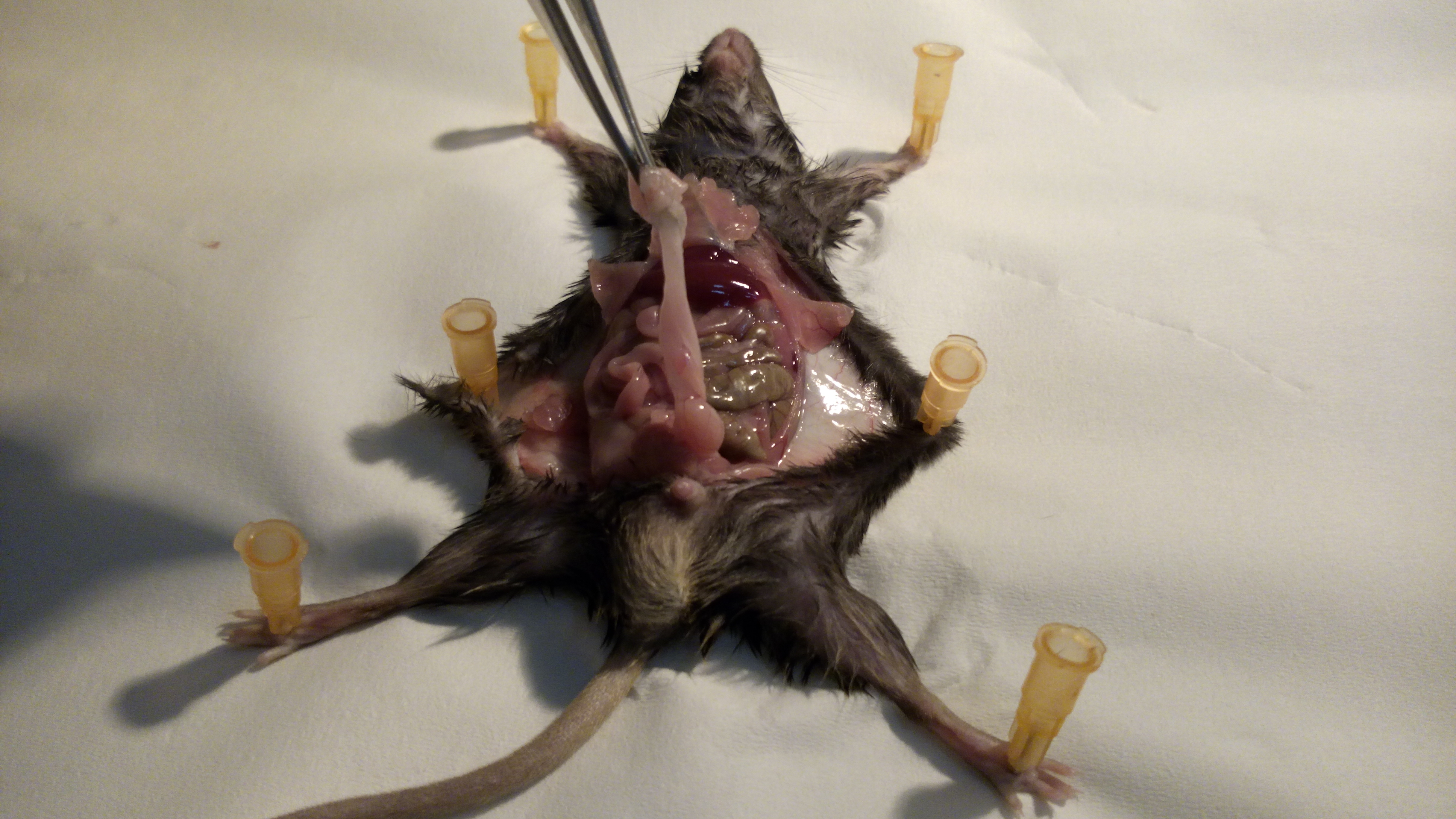
Figure 3. Identification of the VAT. The perigonadal VAT is located in the lower half of the abdomen, surrounding the gonads. - Cut out the VAT carefully along the epididymis and vas deferens (in males) or along the uterus (in females). Take care not to excise any part of the gonads (Figure 4) (Supplementary Figure 1).
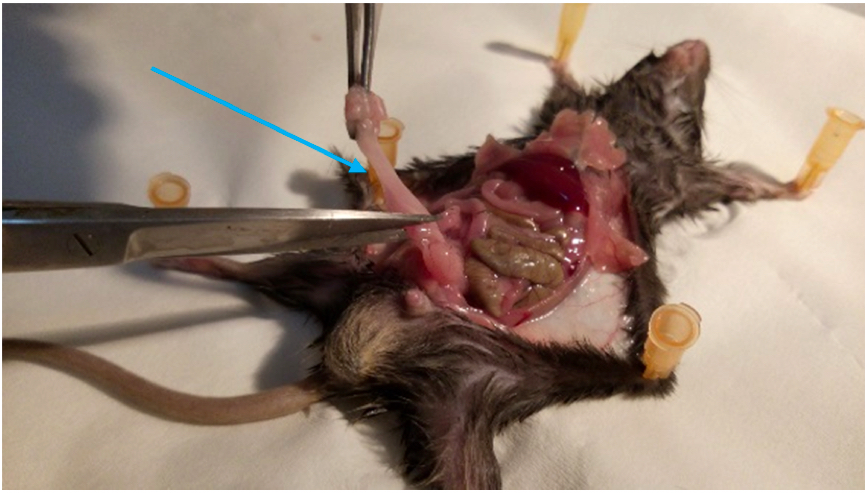
Figure 4. Isolation of VAT. Gently cut out the VAT without damaging the gonads (indicated by arrow). - Slice the VAT into small parts (of approximately 3 mm) with scissors and put them in a 50 ml tube (Figure 5), containing 5 ml of freshly prepared 3% RPMI with 1 mg/ml Collagenase D (or IV).
Note that most collagenases are sold as a mixture of proteins and are not a purified enzyme. Some collagenase batches may therefore contain other protease activity and can digest cell surface proteins. We recommend testing the effects of every collagenase batch on your proteins of interest. For example by comparing the expression of your proteins of interest on splenic lymphocytes by flow cytometry with and without collagenase treatment.
Figure 5. Digestion of the VAT. Place the VAT in 50 ml tube containing 5 ml of freshly prepared 3% RPMI with 1 mg/ml Collagenase D for digestion after cutting it into small pieces using a scalpel or scissors. - Incubate the tissue in a thermostatic shaker for 1 h at 37 °C, shaking at 270 rpm (Figure 6).

Figure 6. Incubation. Place the tubes for digestion in a heated, shaking incubator. - Vortex the tube and add 5 ml of fresh, cold 3% RPMI.
- Centrifuge at 500 x g for 5 min. Remove floating adipocytes using a vacuum pump and afterwards carefully discard the remaining supernatant by inverting the tube (Figure 7).

Figure 7. Removal of supernatant after digestion and centrifugation. The pellet contains leukocytes, stromal cells and remaining erythrocytes, which are collectively called the Stromal Vascular Fraction (SVF). The supernatant contains a liquid phase (with debris) and an oil phase and possibly a fat phase. - In order to eliminate erythrocytes, resuspend the pellet in 1ml of hypotonic solution (Erylysis buffer), vortex and leave for 3 min at room temperature.
- Run the suspension over a 70 µm cell strainer placed on a 2 ml Eppendorf tube containing 500 µl of cold 3% RPMI (Figure 8).

Figure 8. Removal of debris. The suspension is run over a sieve to get rid of debris from connective tissue and lysed erythrocytes. - Vortex briefly and centrifuge at 500 x g for 5 min at room temperature.
- Remove the supernatant and resuspend the pellet in 250 µl 3% RPMI.
- Count viable cells using trypan blue dye exclusion. You can expect between 500,000 and 1,000,000 cells per fat pad.
Representative data
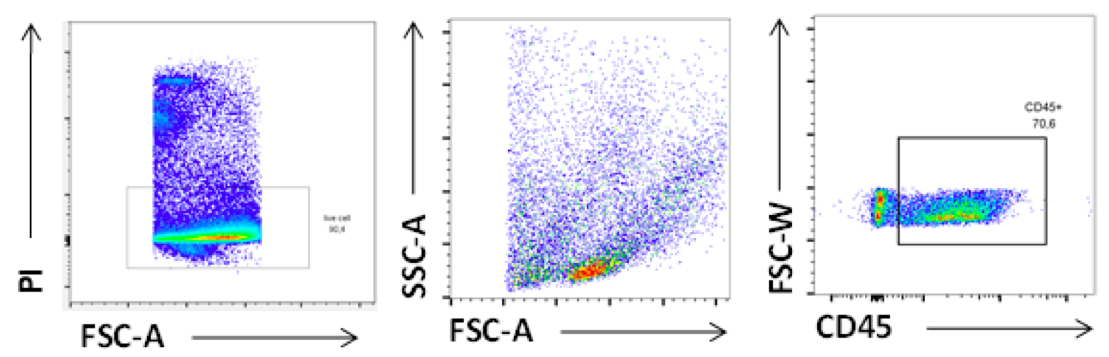
Figure 9. FACS plot of Leukocytes isolated from VAT. Cells were stained with viable dye [Propidium Iodide (PI)] and CD45 antibodies. Gated is for singlets.
Supplementary figures
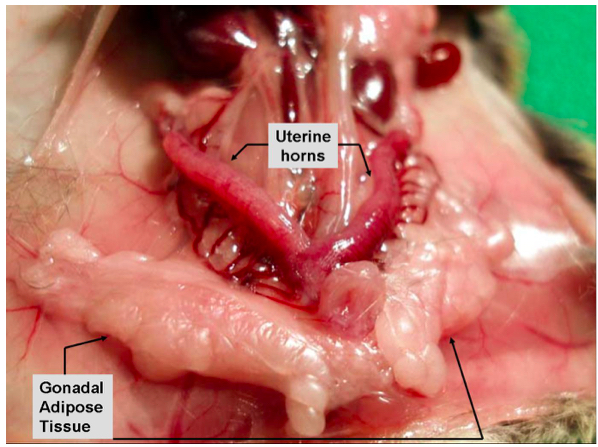
Supplementary Figure 1a. Gonadal/visceral adipose tissue in female mice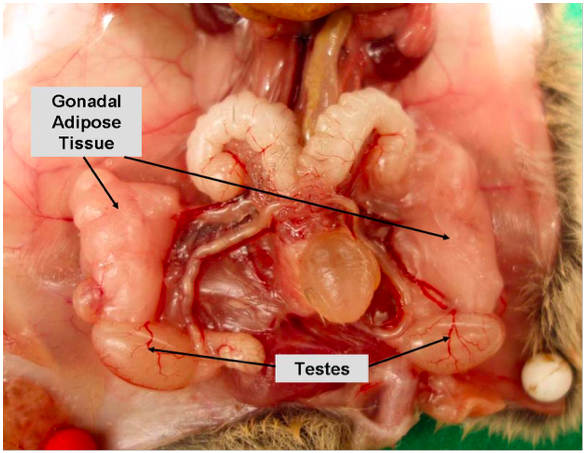
Supplementary Figure 1b. Gonadal/visceral adipose tissue in male mice
Recipes
- 3% RPMI 1640
For 100 ml of buffer, add 3 ml of heat-inactivated FBS to 97 ml RPMI 1640 and
Refrigerate at 4 °C before use - 3% RPMI 1640 + 10 μg/ml Collagenase IV
For 100 ml of buffer, add 3 ml of heat-inactivated FBS and 1 ml of Collagenase IV (0.1 mg/ml) to 96 ml of RPMI 1640 and Refrigerate at 4 °C before use - Erylysis buffer
500 ml MilliQ water
0.83% NH4Cl
0.168% Na2CO3
1 mM EDTA (pH 7.3)
Sterile filtration - FACS wash buffer (pH 7.0-8.0)
1x PBS (pH 7.3)
0.2% BSA
1 mM EDTA
Acknowledgments
When using this protocol, please refer to Wensveen et al. (2015). This work was supported by the European Foundation for the Study of Diabetes (New Horizons Program), the Unity through Knowledge Fund (15/13 to B. P.), the University of Rijeka (13.06.1.1.03 to B. P.), the EU ESFEuropean Social Fund - ES (HR.3.2.01-0263 to B.P.), the Netherlands Organization for Scientific Research (91614029 to F. M. W.) and the European Commission (PCIG14-GA-2013-630827 to F. M. W.).
References
- Ng, A. C., Wai, D. C., Tai, E. S., Ng, K. M. and Chan, L. L. (2012). Visceral adipose tissue, but not waist circumference is a better measure of metabolic risk in Singaporean Chinese and Indian men. Nutr Diabetes 2: e38.
- Wajchenberg, B. L. (2000). Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21(6): 697-738.
- Wensveen, F. M., Jelencic, V., Valentic, S., Sestan, M., Wensveen, T. T., Theurich, S., Glasner, A., Mendrila, D., Stimac, D., Wunderlich, F. T., Bruning, J. C., Mandelboim, O. and Polic, B. (2015). NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol 16(4): 376-385.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Valentić, S., Wensveen, F. M. and Polić, B. (2015). Isolation of Lymphocytes from Murine Visceral Adipose Tissue. Bio-protocol 5(23): e1669. DOI: 10.21769/BioProtoc.1669.
Category
Immunology > Immune cell isolation > Lymphocyte
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link