- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Excision of Visceral Adipose Tissue from Live Mice (VATectomy)
Published: Vol 5, Iss 23, Dec 5, 2015 DOI: 10.21769/BioProtoc.1668 Views: 14297
Reviewed by: Shanie Saghafian-HedengrenAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2530 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2552 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3991 Views
Abstract
The visceral adipose tissue (VAT) has been shown to play an important role in various biological functions. It is a storage depot for nutrients and it is an important endocrine organ producing hormones that control systemic metabolism (McGown et al., 2014). Importantly, following diet-induced obesity, VAT accumulates a large number of activated immune cells, which produce cytokines that drive chronic systemic inflammation and promote insulin resistance (Johnson et al., 2012; Wensveen et al., 2015). VAT therefore plays a key role in metabolic, endocrinological and immunological research. To show the importance of this organ in various research models, one may surgically remove the organ in a procedure called VATectomy. This protocol describes the technical procedures required for an efficient VATectomy of the perigonadal fat pads in mice.
Keywords: Visceral Adipose TissueMaterials and Reagents
- Male Mouse (e.g., C57BL/6, typically of 6-12 weeks old) (male mice are more severely affected by type 2 diabetes than female mice, and they are used exclusively in diet-induced diabetes studies) (Singer et al., 2015).
- Skin disinfectant [e.g., Chlorhexidine gluconate (generic name: Pliva®sept pjenušavi) (PLIVA HRVATSKA, catalog number: 536-02884 )
- Non-Steroid anti-inflammatory drugs (NSAIDs) [e.g., Meloxicam (generic name: loxicum) (5 mg/ml) (Norbrook Laboratories, catalog number: SC-200626 )]
- Inhalation anesthetics [e.g., Forane (generic name: isofluranum) (Abbott Laboratories, catalog number: B506 ).
- Sterile physiological salt solution (0.9% NaCl)
- Depilation cream (Depilation) (Afrodita kozmetika, catalog number: 05-506 )
- Meloxicam working solution (see Recipes)
Equipment
- Inhalation anesthesia equipment (alternatively, one may use intravenous anesthesia)
- Basic surgical instruments (e.g., needle holder, tweezers, scissors, scalpel, hemostat, retractor, surgical needle)
- (Absorbable) suture materials [e.g., Coated VicrylTM, size 3-0 (Ethicon, model: JB944 ) or stapler [e.g., Reflex, 9 mm applier (AgnTho's, model: 202-1000 )]
- Hair trimmer
- Scale
Procedure
- To reduce the amount of stress experienced by the animals as a result of pain, we recommend injecting animals intraperitoneally with NSAIDs, 30 min before starting the surgical procedure. Weigh mice and apply the dosage per kg of bodyweight as indicated (for meloxicam, use 1 mg/kg dissolved in 0.9% NaCl) (Ramsey, 2014).
- Place mice in the anesthetic chamber. Adjust the flow of anesthetics until the animals do not respond to a stimulus that triggers the pain reflex (i.e., pinching the toes). Typically, this requires a 2-4% Isoflurane gas mixture, with 2 L/min of oxygen flow. Visually, one will observe a loss of tail-righting and a reduced breathing rate. Typically it takes approximately 1 min for the mice to be sufficiently anesthetized (Figure 1).

Figure 1. Anesthesia of animals. Mice are placed in an anesthesia chamber (ideally red transparent) and oxygen/inhalation anesthetic flow is controlled. - When mice are anesthetized, remove the hair from the abdomen (in the umbilical and hypogastric regions along the linea alba) using a trimmer (Figure 2). When working efficiently, one can do this without continuous administration of anesthesia gas, as the animal will take about 60 sec before it regains responsiveness. Alternatively, one can use depilation cream. When using cream, take care to properly clean the animals after application to avoid skin irritation.
- Place the animal back in the anesthetic chamber and wait until the animal is again completely anesthetized.
- Place the mouse with its back on the operation table and apply the nose cone that provides the inhalation anesthesia to the mouse (Figure 2). Reduce the isoflurane rate to 1.5-2% with 2 L/min of oxygen flow. The operation table should be placed in a biosafety cabinet to ensure a sterile working condition.

Figure 2. Proper placing of the animal for surgery. Place the animal in such a way that the abdomen is easily accessible, whilst ensuring proper anesthesia. The blue arrow indicates where mice need to be shaved [i.e., in the hypogastric region (down from the umbilicus) along the linea alba]. - Disinfect the skin.
- Use a scalpel to make a Pfannenstiel incision in the skin along the linea alba in the hypogastric region (down from the umbilicus).
- Next, make a cut (0.5 cm) into the muscle and peritoneum (Figure 3).
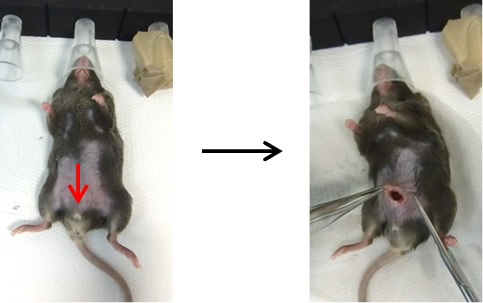
Figure 3. Opening of the abdomen. Pull up the skin with tweezers and cut the abdominal wall, making sure to cut skin, muscle and peritoneum, without damaging the underlying internal organs. The red arrow indicates where mice need to be cut. - There are two perigonadal adipose tissues. Pull them out one by one with tweezers and make an incision at the basis of adipose tissue along the epididymis and testis to completely remove the tissue (Figure 4). For sham-operated controls, pull out the fat pads and place them back inside the abdomen one by one (Video 1). Video 1. Vasectomy and sham operation
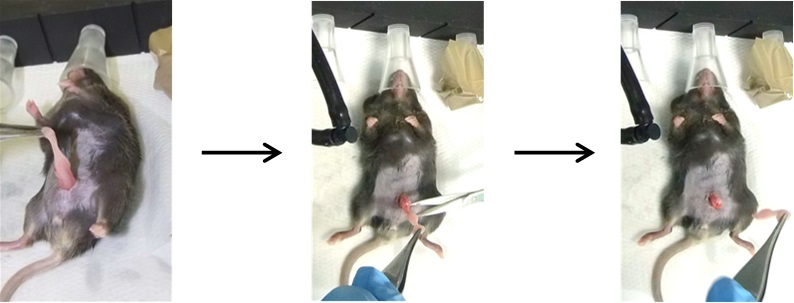
Figure 4. Removal of the VAT. Gently pull out the abdominal fat pads without touching the gonads and cut the VAT along the gonads. - Sew all three layers (peritoneum, muscle and skin) together (Figure 5). Preferably, use an absorbable fiber. Alternatively, non-absorbable fiber or a surgical suture stapler can be used (Singer et al., 2015; Boyer, 2014.).
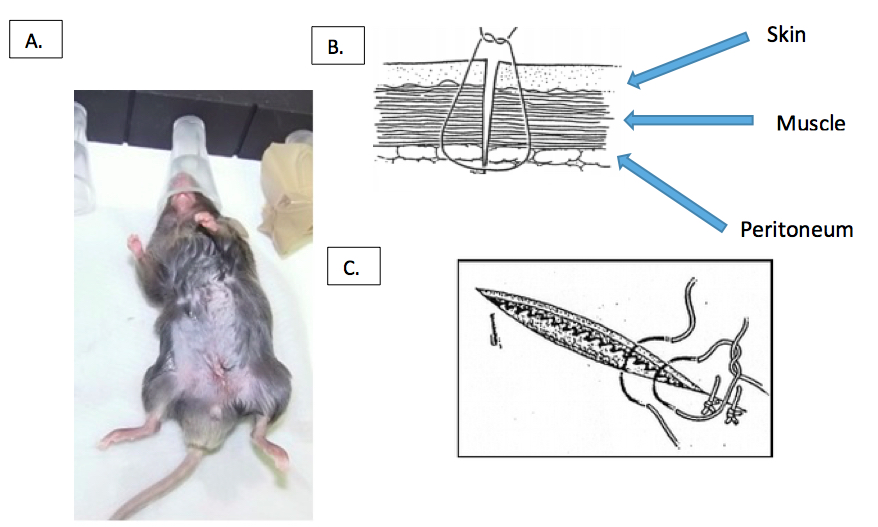
Figure 5. Closing of the abdomen. A. Close the abdomen with one or two stitches. B. sagittal and C. frontal illustration of layers (peritoneum, muscle and skin) and where the sutures should be placed in relation to these layers (Boyer, 2014). - Inject mice with 1 ml of sterile physiological salt solution intraperitoneally to compensate for loss of liquids.
- After surgery, mice will be treated twice daily with NSAIDs for three days.
- If non-absorbable sewing materials have been used, remove these ten days after surgery.
- Fourteen days after surgery, additional experiments can be started. At this time, the site of surgery will have partially been overgrown with new hair.
Notes
- All experiments using mice were approved beforehand by your Institutional Animal Care and Use Committee and were in accordance with national and international guidelines.
- Laboratory rodents do not have a vomiting reflex. Therefore, fasting prior to start of the surgical procedure (which would be advised for larger animals) is not required.
- A skilled surgeon can complete one VATectomy within 5 min. In this period, a warming mat is not required. Unskilled people are advised to use a warming mat to prevent hypothermia of the animals.
- We advise that people without prior surgical experience practice the procedure on a euthanized animal before attempting to perform surgery on a living mouse.
Recipes
- Meloxicam working solution
- Meolxicam (5 mg/ml) 1 ml
- Physiological salt solution (0.9% NaCl in H2O) 9 ml
Acknowledgments
When using this protocol, please refer to Wensveen et al. (2015). This work was supported by the European Foundation for the Study of Diabetes (New Horizons Program), the Unity through Knowledge Fund (15/13 to B. P.), the University of Rijeka (13.06.1.1.03 to B. P.), the European Social Fund - ES (HR.3.2.01-0263 to B. P.), the Netherlands Organization for Scientific Research (91614029 to F. M. W.) and the European Commission (PCIG14-GA-2013-630827 to F. M. W.).
References
- Boyer, L. (2014). A student guide to wound closure. (On date 4 of Septemer 2015).
- Johnson, A. R., Milner, J. J. and Makowski, L. (2012). The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev 249(1): 218-238.
- McGown, C., Birerdinc, A. and Younossi, Z. M. (2014). Adipose tissue as an endocrine organ. Clin Liver Dis 18(1): 41-58.
- Ramsey, I. (2014). BSAVA Small animal formulary Vol 8th edition. BSAVA, UK.
- Singer, K., Maley, N., Mergian, T., DelProposto, J., Cho, K. W., Zamarron, B. F., Martinez-Santibanez, G., Geletka, L., Muir, L., Wachowiak, P., Demirjian, C. and Lumeng, C. N. (2015). Differences in hematopoietic stem cells contribute to sexually dimorphic inflammatory responses to high fat diet-induced obesity. J Biol Chem 290(21): 13250-13262.
- Wensveen, F. M., Jelencic, V., Valentic, S., Sestan, M., Wensveen, T. T., Theurich, S., Glasner, A., Mendrila, D., Stimac, D., Wunderlich, F. T., Bruning, J. C., Mandelboim, O. and Polic, B. (2015). NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol 16(4): 376-385.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Šestan, M., Wensveen, F. M. and Polić, B. (2015). Excision of Visceral Adipose Tissue from Live Mice (VATectomy). Bio-protocol 5(23): e1668. DOI: 10.21769/BioProtoc.1668.
Category
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link