- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Permanent Occlusion of the Left Anterior Coronary Artery in the Rat
Published: Vol 5, Iss 22, Nov 20, 2015 DOI: 10.21769/BioProtoc.1663 Views: 9309
Reviewed by: Ivan ZanoniMartin V KolevAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Morphological Evaluation of Wound Healing Events in the Excisional Wound Healing Model in Rats
Lígia Reis de Moura Estevão [...] Joaquim Evêncio-Neto
Jul 5, 2019 12897 Views

Maternal Immune Activation with the Viral Mimetic Poly:IC in Pregnant Rats
Thaisa Meira Sandini [...] John George Howland
Nov 20, 2020 4173 Views

Using the Cecal Ligation and Puncture Model of Sepsis to Induce Rats to Multiple Organ Dysfunction
Jose Manuel Condor Capcha [...] Samirah A. Gomes
Apr 5, 2021 5808 Views
Abstract
Left ventricular (LV) remodeling occurs in many patients after myocardial infarction (MI). LV remodeling is characterized by progressive ventricular dilatation and contractile dysfunction, consequently to cardiomyocyte hypertrophy and fibrosis. Despite reperfusion therapies, this pathophysiological process is the main cause of cardiac evolution toward heart failure. Moreover, the outcome of patients after MI is largely dependent on the initial cardiac injury. Thus, this is of major clinical interest to develop new pharmacological strategies to limit infarct size and prevent or reverse left ventricular remodeling. Such preclinical cardiovascular treatments are often tested in rodents. The rat model of myocardial infarction is commonly used. In this model, the permanent ligation of the left anterior descending coronary artery is performed (Bousquenaud et al., 2013a).
After being used to this surgical technique and experimented, the operator will need 20 min per rat from the anesthesia to the rat recovering.
Materials and Reagents
- Sterile drapes
- Non-resorbable silk: Prolene 7.0 (Ethicon, catalog number: F1839 )
- Resorbable silk: Vicryl 4.0 (Ethicon, catalog number: V134 )
- Syringes (BD Biosciences, catalog number: 309659 )
- 2 shoelaces (around 20 cm)
- Tracheal tube (Fine Science Tools, catalog number: RSP-ETT1605 )
- Endotracheal tube (Kent Scientific Corporation, catalog number: RSP-ETT1605 )
- Rat Adult male Wistar rats weighing around 300 g (Charles River Laboratories International)
- Dermic Betadine 10%
- Antibiotics: Amoxicilline (Clamoxyl 100 mg/kg/24 h)
- Trichrome stain (Masson) Kit (Sigma-Aldrich, catalog number: HT15 )
- Anti-sarcomeric alpha actinin antibody [EA-53] (Abcam, catalog number: ab9465 )
Equipment
- Gaseous anesthesia delivery system (Minerve)
- Ventilator (Kent Scientific Corporation, model: TOPO220 )
- Fine curved forceps (Fine Science Tools, catalog number: 11272-30 )
- Shaver (Kent Scientific Corporation, catalog number: CL8787 )
- Rasor blade (Fine Science Tools, catalog number: 10008-13 )
- Scalpel (Fine Science Tools, catalog number: 10011-00 )
- Needle holders (Fine Science Tools, catalog number: 12001-13 )
- Retractor (Fine Science Tools, catalog number: 17012-11 )
Procedure
- Anesthesia
- Induce a profound anesthesia while providing a mixture of 3.5% isoflurane and 1.5% oxygen in room air. A high percentage of isoflurane is chosen in order to induce a profound anesthesia as a thoracotomy needs to be performed.
- Place the rat in the induction chamber. Cover the box with a tissue to put the animal in a dark environment (to reduce the stress).
- Set the ventilation system at 50 respiratory cycles per minute. The pressure is set at 30 mmHg and the tidal volume at 2.5/3 ml per cycle; according to the rat weight (0.5 ml/100 g body weight). Pressure must not exceed 2 mmHg. These parameters have been set for use with the Rat.
- Plug the tracheal tube to the ventilation system, the pressure inside must not exceed 5 to 6 mmHg, if not clean the tube.
- As soon as deep anesthesia is reached (respiratory frequency inferior to 50 cycles per minute and loss of footpad reflex), open the aspiration system and take out the rat from the induction chamber.
Pull out the rat tongue with a fine curved forceps and with the help of a light find out the trachea aperture. Insert the tracheal tube in the trachea.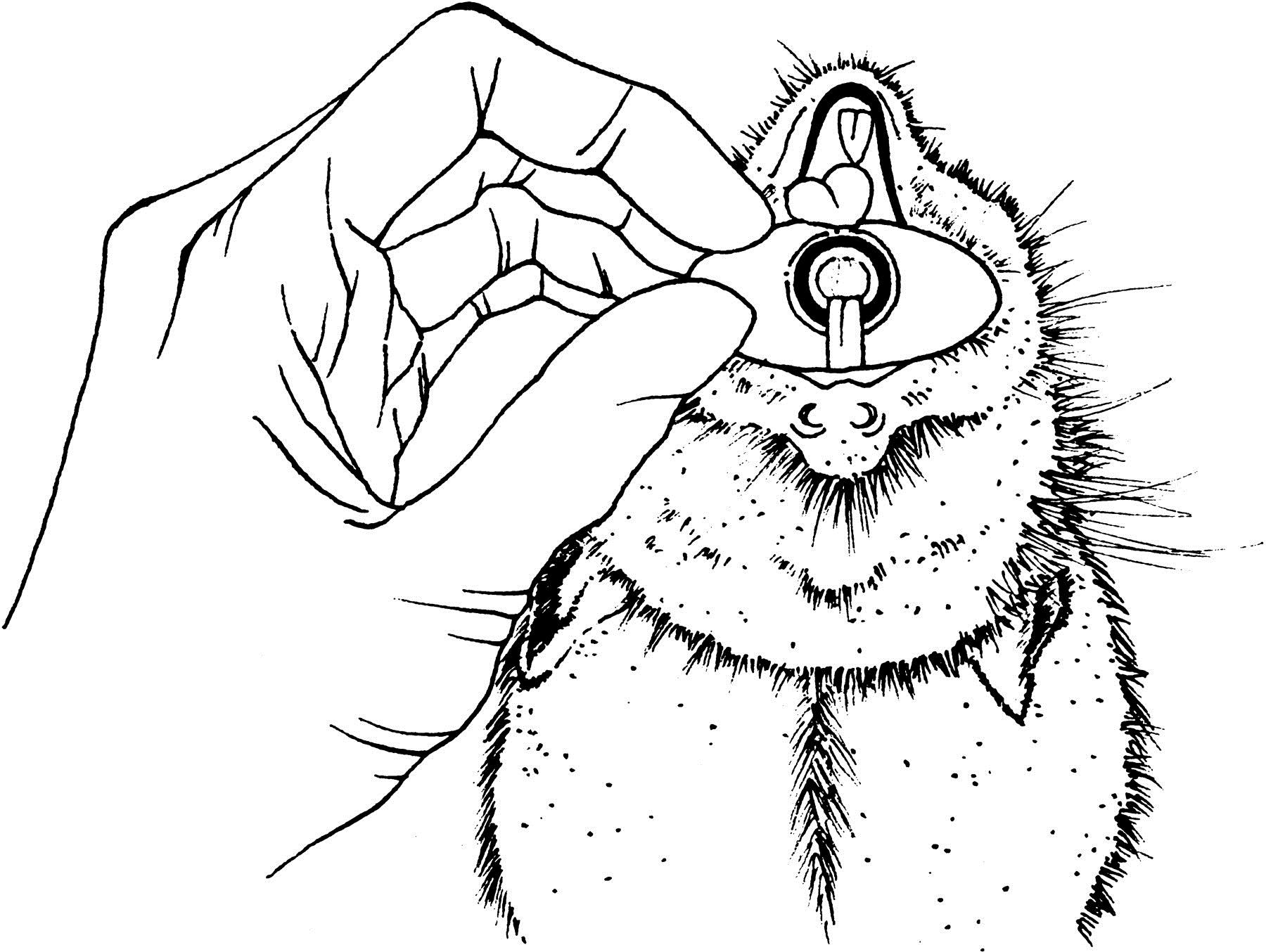
Figure 1. The anatomy of the rat mouth - Quickly plug the tracheal tube to the ventilation system. Be certain that there was no wrong way by checking the lungs swelling. If the esophagus has been intubated, the operator will clearly notice the stomach inflation and must remove the tube immediately.
- In case the rat has not been intubated correctly, the operator must let it completely recovering from anesthesia before restarting the procedure from step A1.
- Attach the tracheal tube to the tooth of the animal, then to its snout.
- Induce a profound anesthesia while providing a mixture of 3.5% isoflurane and 1.5% oxygen in room air. A high percentage of isoflurane is chosen in order to induce a profound anesthesia as a thoracotomy needs to be performed.
- Surgery
- Place the rat in dorsolateral recumbency and place the light on top of it.
- Attach the left anterior foot of the animal in order to stretch it.
- Shave the left part of the thorax and sternum.
- Disinfect the skin with Betadine.
- Cut the skin at an angle of 45° going from the sternum.
- Using a round-tip tweezer, detach the skin from the more superficial muscle layer (pectoralis major), and then detach it from the deeper muscle layer (serratus anterior).
- Strongly hold the 6th rib and cut the intercostal muscle in between the 6th and the 5th rib, inducing thoracotomy. Insert the retractor between the 6th and 5th rib.
- Proceed to pericardiotomy with the fine curved forceps: start from the apical part of the pericardium to make free the left ventricle.
- Using the permanent silk, proceed to the ligation of the left anterior coronary artery. Go in the ventricular muscle with a rotation movement, this allowing staying inside the muscle and avoiding penetrating inside the ventricle.
- Decrease the isoflurane flux to 1.5%.
- Remove the retractor and put the drain inside the cardiac cavity.
- Stop the anesthesia.
- Close the thorax: Join the 2 ribs with the non-permanent silk. Then close the muscle layer. Close the second muscle layer and then the skin.
- Drain the cavity and clean the skin with Betadine.
- Place the rat in dorsolateral recumbency and place the light on top of it.
- Post-surgical care and recovery
- Administrate the antibiotic by intramuscular injection (200 mg/kg) every 48 h and during one week.
- Make the animal left foot free and remove the tracheal tube.
- Stop the ventilation, but check the recovery of the animal. If it stops breathing, immediately put on back the ventilation. Do it until the animal has completely recovered a strong breathing and starts to show discomfort with the tube.
- Remove the tube and at the same time aspirate the mucus with the syringe.
- Place the rat on dorsal recumbency under heather and check it until it is completely awake.
- Administrate antibiotic 24 h and 48 h later.
- Mortality is very low after 48 h.
- Administrate the antibiotic by intramuscular injection (200 mg/kg) every 48 h and during one week.
Representative data
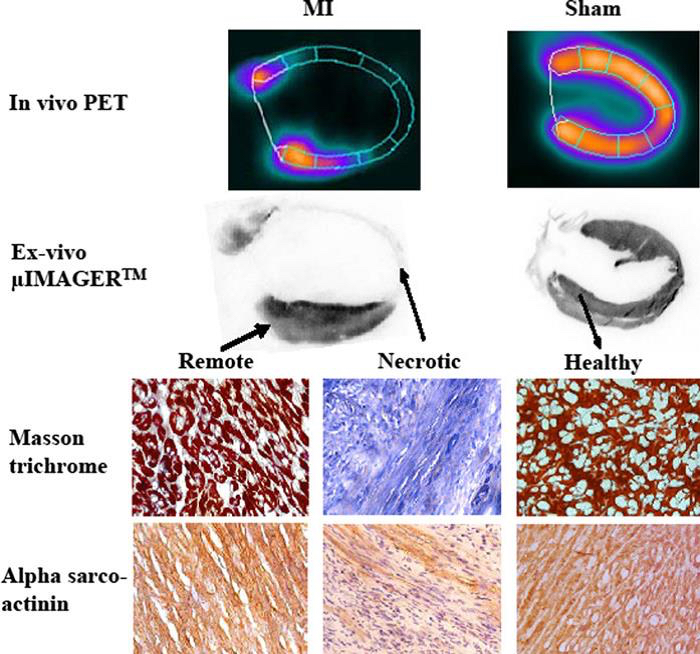
Figure 2. Illustration of the ex vivo characterization of an infarcted and a healthy rat heart; described by in vivo positron emission tomography (PET). 18F-fluorodeoxyglucose (FDG)-PET is a reference cardiac imaging technique in man and rat, because of its ability to accurately differentiate healthy from necrotic myocardium based on cardiomyocytes metabolic activity. (1) Vertical long-axis slices (upper half) recorded in the same rat in vivo by Fluorodeoxyglucose-PET and ex vivo by µIMAGERTM. (2) Immunohistochemical data (lower half) showing fibrosis development (blue color at Masson trichrome) and the decrease in cytoskeleton elements (alpha-sarco actinin staining in brown color) within the infarcted segments. Pictures from a rat with large MI and a sham-operated rat are shown. Magnification *40. (Bousquenaud et al., 2012)
Notes
- By its shape and diameter, the anatomy of the rat left anterior coronary artery displays a strong inter-individual variability. Thus, regardless of the accuracy and level of experience of the manipulator, this surgical model provides a variable extent of myocardial infarct. This will have to be taken into account when planning the number of animals to use in order to get significant results.
- The survival of the animals is very dependent on the speed of recovery after surgery. It is recommended to start decreasing the isoflurane flux when starting to stitch up the muscles, and stop the anesthesia as soon as the skin wound is closed. This can be performed only when the experimenter works rapidly, since the recovery from isoflurane anesthesia is fast.
Acknowledgments
This work was supported by grants from the National Funds of Research, the Society for Research on Cardiovascular Diseases, the Ministry of Culture, Higher Education and Research of Luxembourg, and the ‘‘Fondation de France’’.
References
- Bousquenaud, M., Maskali, F., Poussier, S., Marie, P. Y., Boutley, H., Karcher, G., Wagner, D. R. and Devaux, Y. (2012). Acipimox-enhanced (1)(8)F-fluorodeoxyglucose positron emission tomography for characterizing and predicting early remodeling in the rat infarct model. Int J Cardiovasc Imaging 28(6): 1407-1415.
- Bousquenaud, M., Maskali, F., Poussier, S., Zangrando, J., Marie, P. Y., Boutley, H., Fay, R., Karcher, G., Wagner, D. R. and Devaux, Y. (2013a). Cardioprotective effects of adenosine within the border and remote areas of myocardial infarction. EJNMMI Res 3(1): 65.
- Bousquenaud, M., Wagner, D. R., Maskali, F., Marie, P. Y. and Devaux, Y. (2013b). Long-term survival after a massive left ventricular infarction evidenced by FDG-PET and leaving intact only the septal wall. Int J Clin Exp Med 6(1): 84-85.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bousquenaud, M., Marie, P. and Devaux, Y. (2015). Permanent Occlusion of the Left Anterior Coronary Artery in the Rat. Bio-protocol 5(22): e1663. DOI: 10.21769/BioProtoc.1663.
Category
Immunology > Animal model > Rat
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










