- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
13C Tracer Studies of Metabolism in Mouse Tumor Xenografts
Published: Vol 5, Iss 22, Nov 20, 2015 DOI: 10.21769/BioProtoc.1650 Views: 12869
Reviewed by: Masahiro MoritaShannon RuppertYong Teng

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Hypoxia Studies with Pimonidazole in vivo
Kristina Y. Aguilera and Rolf A. Brekken
Oct 5, 2014 28625 Views

Stable Isotope Resolved Metabolomics Studies in ex vivo TIssue Slices
Teresa W-M. Fan [...] Richard M. Higashi
Feb 5, 2016 11729 Views

Analysis of Leukemia Cell Metabolism through Stable Isotope Tracing in Mice
Nick van Gastel [...] David T. Scadden
Oct 5, 2021 5229 Views
Abstract
Mice are widely used for human tumor xenograft studies of cancer development and drug efficacy and toxicity. Stable isotope tracing coupled with metabolomic analysis is an emerging approach for assaying metabolic network activity. In mouse models there are several routes of tracer introduction, which have particular advantages and disadvantages that depend on the model and the questions addressed. This protocol describes the bolus i.v. route via repeated tail vein injections of solutions of stable isotope enriched tracers including 13C6-glucose and 13C5,15N2-glutamine. Repeated injections give higher enrichments and over longer labeling periods than a single bolus. Multiple injections of glutamine are necessary to achieve adequate enrichment in engrafted tumors.
Keywords: SIRMMaterials and Reagents
- 0.5 ml K2-EDTA collection tubes (BD, catalog number: 363706 )
- Sterile syringes and gauge 30 needles (Thermo Fisher Scientific, catalog number: 10142534 )
- 5-inch squares Al foil
- Indelible marker pens (black)-Sharpie fine tip
- Pasteur pipet
- Lancets (MEDIpoint, Inc., model: Goldenrod 5 mm )
- Mice bearing tumor xenograft
Note: The strain of immunocompromised recipient mouse depends on type of implant. Young NOD/SCID Gamma (NSG) mice are especially favorable for the patient derived xenograft (PDX) model. - Ketamine for anesthesia if needed, Schedule III controlled substance (Sigma-Aldrich, catalog number: K2753 )
- Tracer [e.g., 13C6-glucose, 13C2-1, 2-glucose (60 mg/mouse), or 13C5, 15N2-glutamine (22 mg/mouse)]
- Sources
- D-Glucose-13C6 (Cambridge Isotope Laboratories, Inc., catalog number: CLM-1396-CTM )
- D-Glucose-1, 2-13C2 (Cambridge Isotope Laboratories, Inc., catalog number: CLM-504 )
- L-Glutamine-13C5, 15N2 (Cambridge Isotope Laboratories, Inc., catalog number: CNLM-1275 )
- D-Glucose-13C6 (Cambridge Isotope Laboratories, Inc., catalog number: CLM-1396-CTM )
- Liquid nitrogen
- 70% ethanol
- 10% Neutral buffered formalin (Thermo Fisher Scientific, ProtocolTM, catalog number: 032-059 )
- 25% w/v sterile filtered (0.2 µm) 13C glucose in PBS
Note: Stock solution can be frozen and stored at -25 °C. - Sterile wipes (Uline, catalog number: S-16183 )
- Sodium Chloride (NaCl) (Thermo Fisher Scientific, catalog number: S271-1 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Sodium phosphate dibasic (Na2HPO4) anhydrous (Sigma-Aldrich, catalog number: S-0876 )
- Potassium phosphate monobasic (KH2PO4) anhydrous (Sigma-Aldrich, catalog number: P9791 )
- 10x Phosphate Buffered Saline (PBS) (see Recipes)
- Stock solution for 0.2% 13C6-glucose (see Recipes)
- Stock solution for 13C5, 15N2-Gln (10 ml) (see Recipes)
Equipment
- Mouse restraining system (Plas-Labs, Inc., model: 541-RR )
- FirstHand Surgical Instrument Kits for Mice and Rats (Kent Scientific Corporation, model: INSMOUSEKIT )
- Blue wax dissection tray (VWR International, catalog number: 10060-188 )
- Warming blanket (Kent Scientific Corporation, model: DCT-15 )
- Water bottle with DI water (Thermo Fisher Scientific, catalog number: 02-897-11 )
- Polystyrene Weigh boats (USA Scientific Inc., catalog number: 2347-1427 )
- Calipers for measuring tumor size (Thermo Fisher Scientific, catalog number: 15-077-957 )
- 2-place Balance for weighing tissues (VWR International, Adventurer Ohaus®, catalog number: 10153-744 )
- Refrigerated Microfuge (Eppendorf, model: 5417R with rotor)
- Dewar/bucket (Nalgene plastic dewar) for lN2 (Thermo Fisher Scientific, catalog number: S34074C )
- Large Styrofoam box
Procedure
All experiments must be performed under an IACUC approved protocol. For human tissue xenografts, the appropriate IRB approvals must also be met.
- Schedule
- Allow tumor in mice to develop to at least 0.5 cm and up to 1.5 cm according to specific protocols for ectopic or orthotopic xenografts of cells or fresh tissue (PDX model) as well as other tumor models (e.g., transgenic tumor model).
- Preparations
The day before Stable Isotope Resolved Metabolomics (SIRM) experiment:- Pre-label Al squares with date, mouse number and tissue (e.g., tumor), and blood collection tubes (2 per mouse for collection immediately after tracer injection, and at the time of necropsy) with date, mouse number, collection time.
- Label storage box for -80 °C freezer.
- Prepare tracer solutions e.g., 25% 13C6-glucose (1.344 M) or 36.2 mg/ml 13C5, 15N2-Gln (0.2 M) in sterile PBS, sterile filter, and store in aliquots at < -20 °C.
- Label formalin bottles with date, mouse number and tumor source.
- Assemble all components in sealable boxes for transport.
- Prepare schedule sheet (see example below, Table 1).
- Pre-label Al squares with date, mouse number and tissue (e.g., tumor), and blood collection tubes (2 per mouse for collection immediately after tracer injection, and at the time of necropsy) with date, mouse number, collection time.
- Tracer Injection
When mice are ready, restrain the mouse in the mouse restrainer, sterilize the injection area of the tail vein using a sterile wipe. Inject stable isotope tracers through the tail vein (or other convenient vein, such as submandibular vein) into individual immobilized mice*. Placing the mouse on a warming blanket or gently heating the tail visibly dilates the vein to make injection easier.- Record time of injection.
- Immediately take blood sample (see step 5 below).
- For 13C6-glucose (can be purchased from Sigma Isotec or Cambridge Isotopes Laboratories, see above): Inject 80 µl (20 mg) each 25% (w/v) stock solution (in PBS and 0.2 µm sterile filtered) at 15 min intervals 3 times (total = 322 µmol).
- For 13C5, 15N2-Gln: Same as above except for injecting 200 µl (7.2 mg) each 36.2 mg/ml stock solution (in PBS and 0.2 µm sterile filtered) at 15 min intervals 3 times (total = 142 µmol).
- The bolus injections take a few seconds. The extremely high heart rate of mice (500-600 beats per minute for resting adult mice) ensures that the tracer is systemically distributed very rapidly, therefore approximating a pulse.
Note: *We have tried injection into ketamine-anesthetized mice. Since anesthesia slows metabolism, the timing has to be lengthened. Anesthesia can also alter metabolism. With the physical restraint method, it is important to minimize stress to the restrained mice during injection. Black mice are more difficult to inject via tail vein. The ability to do timed injections reproducibly takes considerable practice.
- Record time of injection.
- Tissue harvest
- At 45 min after the first injection (15 min after the last injection), take photographs of the mouse and the tumor.
- Take blood sample before killing mice by cervical dislocation (do not use CO2 asphyxiation or lethal injection of barbiturates as they interfere with metabolism)
- Measure tumor size using calipers (Figure 1).
- Dissect relevant tissues, e.g., tumor, lung, liver, heart, kidney, brain, skeletal muscle, adipose, lower bowel and flash freeze tissues immediately (within 5 min of necropsy) in liquid N2, followed by placing frozen tissues in screw-cap 2 ml microfuge tubes pre-equilibrated in liquid N2 or in aluminum foil (made into a boat shape) floating on liquid N2, followed by wrapping**.
Note: **Use non-erasable (solvent-resistant) permanent ink Sharpie pens to label sample tubes or aluminum foil to avoid label washout. - The order of dissection is dictated mainly by the major organ of interest, which should be removed first if possible. Dissected tissue is rinsed briefly in cold PBS, blotted and flash-frozen in liquid N2 within 2-6 min of euthanasia.
- Tumor should be rinsed in cold PBS, blotted and weighed before flash freezing in lN2 (Figure 1) and photographed.
- Typically at least three people are needed for this work.
- With multiple injections, it is practical to do three mice as a group, timing injections 5 min apart.
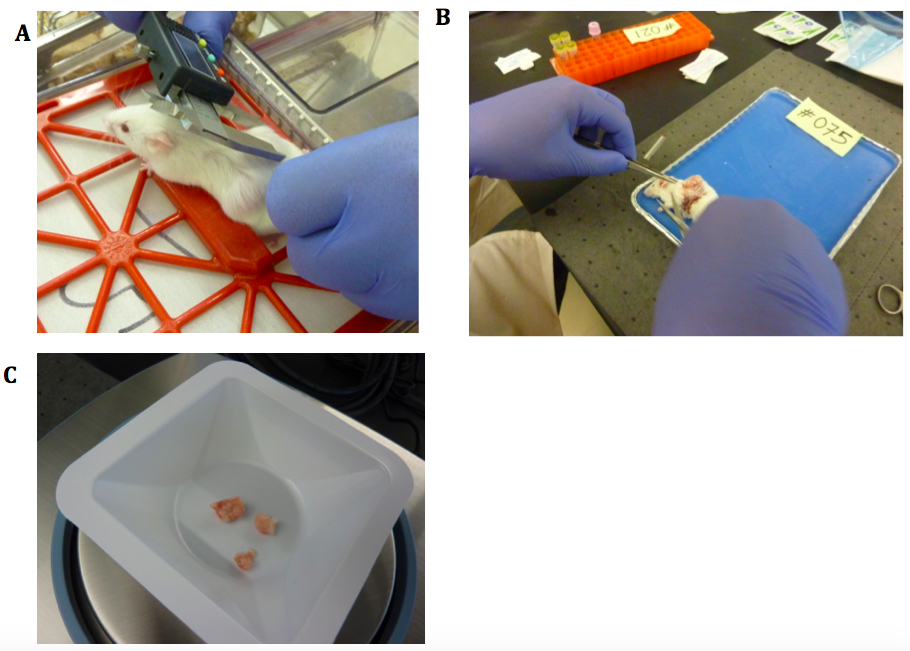
Figure 1. Mouse necropsy. A. NSG mouse with implanted tumors. Measuring size using electronic calipers; B. Dissection to reveal ectopic tumor. Blood tubes in the orange rack; C. Tumor pieces being weighed after blotting.
- At 45 min after the first injection (15 min after the last injection), take photographs of the mouse and the tumor.
- Blood sample processing
- Blood should be collected***immediately into K2-EDTA microtubes ['purple top'] (designed for mice) after tracer injection (1st) and just prior to necropsy (2nd).
- Initial blood collection should be no more than 150 µl; collect as much blood as possible for the 2nd collection.
- Let blood stand at room temperature for 5 min before storage on ice****.
- Centrifuge blood at 3,500 x g for 15 min at 4 °C.
- Separate plasma from blood cells and flash freeze the separated components in liquid N2.
- Blood should be collected***immediately into K2-EDTA microtubes ['purple top'] (designed for mice) after tracer injection (1st) and just prior to necropsy (2nd).
- EDTA anticoagulant is preferred over citrate, heparin etc. as EDTA interferes least with metabolism
- ***We collect blood intraorbitally or via the submandibular vein using a lancet which is generally preferred (Golde et al., 2005); other methods may be applicable (e.g., from other vein) and at sacrifice, cardiac punch for maximal blood collection.
- ****It is important to keep blood at RT for 5 min to reduce hemolysis but store blood on ice thereafter until centrifugation.
- All samples are stored at -80 °C or colder.
- If necessary, samples for analysis should be shipped overnight on dry ice.
- Metabolites are extracted from tissues and blood according to established protocols, and then analyzed by high resolution NMR, GC-MS, and FT-ICR-MS to establish not only the content of metabolites, but also their labeled isotopomer and isotopologue distributions, which represents metabolic transformation from the source tracer to the observed metabolites in the interval between injection and necropsy (Fan et al., 2011; Fan, 2012a; Fan et al., 2012; Lane et al., 2011).
- Allow tumor in mice to develop to at least 0.5 cm and up to 1.5 cm according to specific protocols for ectopic or orthotopic xenografts of cells or fresh tissue (PDX model) as well as other tumor models (e.g., transgenic tumor model).
Representative data
Qualitatively similar 13C metabolite profiles of individual organs have been obtained from these experiments at comparable time points, as estimated from 1-D 1H{13C}-HSQC spectra (e.g., Figure 2) (Fan et al., 2011; Yuneva et al., 2012; Sellers et al., 2015; Xie et al., 2014). The amounts of 13C in each metabolite per mg can be calculated from the intensity of the lactate methyl resonance compared with its concentration independently determined by either 1H NMR or GC-MS. These methods also provide the fractional enrichments of each observable metabolite, as described in Lane et al. (2008). Detailed descriptions of data reduction and analysis are provided in Fan et al. (2011) and Lane et al. (2008).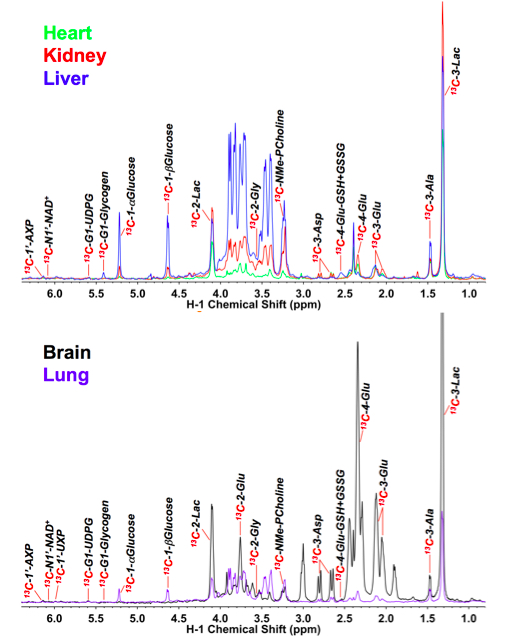
Figure 2. 1D 1H{13C} HSQC spectra of different organs of an NSG mouse infused with 13C6-glucose. The NSG mouse received three boluses of 20 mg each 13C6-glucose via tail vein injection at 15 min interval. Heart, kidney, liver, brain and lung tissues were dissected within 5 min of necropsy, flash-frozen in liq. N2, pulverized in liq. N2, and extracted with acetonitrile: H2O: CHCl3 (4:3:2 v/v) for polar metabolites as described in (10). The NMR spectra were normalized to the residual dry residual weight of the extract. Extracts are lyophilized prior to preparation of the NMR sample, as described (Fan, 2012b).
Table 1. Example record sheet
Title:
Date:
Experiment: Tumor from patient # (Date) implanted into 2 NSG mice (name of researcher).
2 mice, each piece subQ implanted on both flanks
mouse 1 F tumor sizes= ear tag #
mouse 2 M tumor sizes= ear tag #
SIRM/harvesting Date
Treatment: 3 x 80 µl 25% 13C6-glucose tail vein 15 min intervals (M1) or 3 x 200 µl, 36 mg/ml 13C5, 15N2-Gln (M2).
Anesthetic Y/N.
Organs extracted sequentially:
Blood, tumor, lung, heart, liver, kidney, brain…
| Time | Process | Time post injection / min | comments |
| M1 | 80 µl Glc | 0 | |
| Blood | |||
| 80 µl Glc | |||
| 80 µl Glc | |||
| blood | |||
| sac | |||
| Tumor | Mass: g | ||
| Lung | |||
| Heart | |||
| Liver | |||
| kidney | |||
| brain | |||
| M2 | 200 µl Gln | 0 | |
| Blood | |||
| 200 µl Gln | |||
| 200 µl Gln | |||
| blood | |||
| sac | |||
| Tumor | Mass: g | ||
| Lung | |||
| Heart | |||
| Liver | |||
| kidney | |||
| brain |
Put sample into formalin for pathology. Place small samples into medium for further propagation in NSG mice and cell culturing.
Notes
- The injections can be achieved with restraints, which is compromised by release of stress hormones. Alternatively the mice can be anesthetized with ketamine, which can slow general metabolism while they are unconscious and alter certain metabolic pathway(s) due to the non-intended effect of the anesthetic agent.
- Tail vein injection of black mice can be challenging. Alternatives are intraperitoneal injection (which has a different time course), feeding or direct infusion via the jugular vein or carotid artery (de Graaf et al., 2011) (http://jaxmice.jax.org/preconditioned/surgical/pricing.html).
- For xenografts a variety of immune compromised strains are available, which can be used according to the study design (http://jaxmice.jax.org/findmice/index.html). Xenografts can be formed after injection of established cancer cells (Fan et al., 2011; Lane et al., 2011), primary cell lines, and fresh tumor tissue (PDX model) (Sellers et al., 2015) either subcutaneously or orthotopically. The amounts tissue needed and the number of cells depends greatly on the source of the tumor or cell line characteristics.
- Blood analysis is crucial to monitoring the amount of tracer delivered and the overall utilization over the period of the experiment. The transient nature of the experiments provides both information about pathways that are operative during the short labeling time period and the relative rates of uptake and utilization of different tissues. This can be refined by time course analysis of a group of mice harvested at different times post injection. Different numbers of injections also can be used to extend or decrease the degree of labeling in different groups of metabolites. Examples of blood metabolite time courses in mice injected with 13C-glucose are in Fan et al. (2011).
- With bolus injections isotopic steady state cannot be reached (de Graaf et al., 2011; Maher et al., 2012), and metabolites with slow turnover do not become significantly labeled. For labeling these metabolites, continuous infusion via an appropriate vein for a longer period (Marin-Valencia et al., 2012) or longer-term tracer feeding can be employed.
- Due to some variability of mice age, handling, and tumor status, it may not be practical to average metabolite values from different individuals. 5 or more mice/group are needed depending on the effect size. Preferably all the same gender should be used, and whenever possible littermates. With PDX models we usually match the mouse gender to that of the tumor donor.
Recipes
- 10x PBS
80 g NaCl
2 g KCl
14.4 g Na2HPO4 anhydrous
2.4 g KH2PO4 anhydrous
Dissolve in 950 ml 18 MOhm water, pH to 7.4, make to 1 L, sterile filter (0.2 µm) - Stock solution for 13C6-glucose (10 ml)
2.5 g 13C6-glucose
1x PBS to a total of 10 ml
Sterile filter (0.2 mm) into sterile screw cap container
Stored at -4 °C - Stock solution for 13C5, 15N2-Gln (10 ml)
0.362 g 13C5, 15N2-Gln
1x PBS to a total of 10 ml
Sterile filter (0.2 mm) 1 ml aliquots into sterile screw cap vials
Stored at -80 °C
Note: Glutamine stock should be made fresh or stored at -20 °C in small aliquots to avoid repeated freezing and thawing. It forms pyroglutamate on storage in solution at neutral pH at higher temperatures.
Acknowledgments
This protocol was modified from previously published studies ( Fan et al., 2011). The work has been supported by NIH Grants 1R01CA118434-01A2, NIH 5R01ES022191-04, NIH 3R01ES022191-04S1 (to TWMF), R01CA-086412 and RO1 CA150947 (to JY), NIH R21CA133688 (to ANL), NIH P01 CA163223 (to ANL, TWMF and JY), and NIH 1U24DK097215-01A1 (to TWMF, ANL), the Kentucky Challenge for Excellence, and the Susan G. Komen Foundation BCTR0503648.
References
- Golde, W. T., Gollobin, P. and Rodriguez, L. L. (2005). A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 34(9): 39-43.
- Fan, T. W., Lane, A. N., Higashi, R. M. and Yan, J. (2011). Stable isotope resolved metabolomics of lung cancer in a SCID mouse model. Metabolomics 7(2): 257-269.
- Fan, T. W., Lorkiewicz, P. K., Sellers, K., Moseley, H. N., Higashi, R. M. and Lane, A. N. (2012). Stable isotope-resolved metabolomics and applications for drug development. Pharmacol Ther 133(3): 366-391.
- Fan, T. W. (2012a). Considerations of sample preparation for metabolomics investigation. Handbook of Metabolomics, 17.
- Lane, A. N., Fan, T. W., Bousamra II, M., Higashi, R. M., Yan, J. and Miller, D. M. (2011). Clinical applications of stable isotope-resolved metabolomics (SIRM) in non-small cell lung cancer. Omics, 15, 173-182.
- Yuneva, M. O., Fan, T. W., Allen, T. D., Higashi, R. M., Ferraris, D. V., Tsukamoto, T., Mates, J. M., Alonso, F. J., Wang, C., Seo, Y., Chen, X. and Bishop, J. M. (2012). The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab 15(2): 157-170.
- Sellers, K., Fox, M. P., Bousamra, M., 2nd, Slone, S. P., Higashi, R. M., Miller, D. M., Wang, Y., Yan, J., Yuneva, M. O., Deshpande, R., Lane, A. N. and Fan, T. W. (2015). Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest 125(2): 687-698.
- Xie, H., Hanai, J., Ren, J. G., Kats, L., Burgess, K., Bhargava, P., Signoretti, S., Billiard, J., Duffy, K. J., Grant, A., Wang, X., Lorkiewicz, P. K., Schatzman, S., Bousamra, M., 2nd, Lane, A. N., Higashi, R. M., Fan, T. W., Pandolfi, P. P., Sukhatme, V. P. and Seth, P. (2014). Targeting lactate dehydrogenase--a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab 19(5): 795-809.
- Lane, A. N., Fan, T. W. and Higashi, R. M. (2008). Isotopomer-based metabolomic analysis by NMR and mass spectrometry. Methods Cell Biol 84: 541-588.
- Fan, T. W. M. (2012b). In: Fan, T. W. M., Lane, A. N. and Higashi, R. M. (eds). The handbook of metabolomics: Pathway and flux analysis, methods in pharmacology and toxicology. Springer Science, 17: 7-27.
- de Graaf, R. A., Rothman, D. L. and Behar, K. L. (2011). State of the art direct 13C and indirect 1H-[13C] NMR spectroscopy in vivo. A practical guide. NMR Biomed 24(8): 958-972.
- Maher, E. A., Marin-Valencia, I., Bachoo, R. M., Mashimo, T., Raisanen, J., Hatanpaa, K. J., Jindal, A., Jeffrey, F. M., Choi, C., Madden, C., Mathews, D., Pascual, J. M., Mickey, B. E., Malloy, C. R. and DeBerardinis, R. J. (2012). Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed 25(11): 1234-1244.
- Marin-Valencia, I., Yang, C., Mashimo, T., Cho, S., Baek, H., Yang, X. L., Rajagopalan, K. N., Maddie, M., Vemireddy, V., Zhao, Z., Cai, L., Good, L., Tu, B. P., Hatanpaa, K. J., Mickey, B. E., Mates, J. M., Pascual, J. M., Maher, E. A., Malloy, C. R., Deberardinis, R. J. and Bachoo, R. M. (2012). Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab 15(6): 827-837.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lane, A. N., Yan, J. and Fan, T. W. (2015). 13C Tracer Studies of Metabolism in Mouse Tumor Xenografts. Bio-protocol 5(22): e1650. DOI: 10.21769/BioProtoc.1650.
Category
Cancer Biology > Cellular energetics > Tumor microenvironment > Metabolism
Biochemistry > Carbohydrate > Glucose
Cell Biology > Cell metabolism > Carbohydrate
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








