- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Skeletal Myogenesis in vitro
Published: Vol 5, Iss 21, Nov 5, 2015 DOI: 10.21769/BioProtoc.1645 Views: 10588
Reviewed by: Kae-Jiun ChangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3937 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2413 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 230 Views
Abstract
Mature skeletal myofibers are elongated and multinucleated cells. Many stem/progenitor cell types, including committed muscle stem (satellite cells) and progenitor (myoblasts) cells, muscle-derived stem cells, myogenic endothelial cells, and mesenchymal stem/stromal cells, have been shown to exhibit skeletal myogenesis under appropriate inductive conditions. Committed muscle stem/progenitor cells and multipotent stem/progenitor cells which have skeletal myogenic capacity can typically be differentiated into skeletal myofibers in vitro following extended low-serum exposure. Differentiated cells exhibit distinct fiber-like elongated morphology with multiple nuclei and express unique muscle molecular markers indicating myogenesis, including desmin (early) and fast- and/or slow-myosin heavy chain (mature).
Materials and Reagents
- Collagen type-I coated plates (sterilized by UV overnight after coating) (the protocol for coating plates is provided by Sigma-Aldrich in the associated product information)
- Myogenic cells in sterile conditions (Refer to Chen et al., 2014 and Gharaibeh et al., 2008 for primary human and mouse cell isolation respectively)
Note: For mouse cells, C2C12 cell line (ATCC, catalog number: CRL-1772) may be used as a positive control. - DMEM high-glucose (Thermo Fisher Scientific, InvitrogenTM, catalog number: 11995 )
- Fetal bovine serum (FBS) (Invitrogen, catalog number: 10437-028 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 10437-028”. - Horse serum (HS) (Invitrogen, catalog number: 26050-088 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 26050-088”. - Chicken embryo extract (CEE) (Accurate Chemical & Scientific Corporation, catalog number: MD-004-D )
- Penicillin-Streptomycin (P/S) (Invitrogen, catalog number: 15140-122 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 15140-122”. - Monoclonal Anti-Desmin antibody produced in mouse (1:100) (Sigma-Aldrich, catalog number: D1033 ), Mouse anti-fast-MyHC (1:250) (Sigma-Aldrich, catalog number: M4276 ) or Monoclonal Anti-Myosin (Skeletal, Slow) antibody produced in mouse (1:250) (Sigma-Aldrich, catalog number: M8421 )
- Normal donkey serum (Jackson Immuno Research, catalog number: 017-000-121 )
- Alexa 594-conjugated anti-mouse IgG antibody (1:500) (Invitrogen, Molecular Probes, catalog number: A-21203 ) or Alexa 488-conjugated anti-mouse IgG antibody (1:500) (Invitrogen, Molecular Probes, catalog number: A-21202 )
Note: Currently, it is “Thermo Fisher Scientific, NovexTM, catalog numbers: A-21203 and A-21202”. - 4',6-diamidino-2-phenylindole (DAPI) (100 ng/ml, diluted with DPBS from the stock) (Sigma-Aldrich, catalog number: D9542 )
- Mouse-on-mouse (M.O.M) Basic kit (staining kit) (Vector Labs, catalog number: BMK-2202 )
- Collagen from calf skin (non-sterile) (Sigma-Aldrich, catalog number: C9791 )
- Dulbecco’s DPBS without calcium and magnesium (DPBS) (Invitrogen, catalog number: 14190-250 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: A-21203 and A-21202”. - Methanol (Sigma-Aldrich, catalog number: 322415 )
- Acetone (Sigma-Aldrich, catalog number: 270725 )
- Formalin (10%) (Sigma-Aldrich, catalog number: HT501128 )
- Trypsin-EDTA (1x, diluted from the 10x stock with sterile DPBS, no phenol red) (Invitrogen, catalog number: 15400-054 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM catalog number: 15400-054”. - Collagen type-I (Sigma-Aldrich, catalog number: C9791)
- Muscle proliferation medium (see Recipes)
- Muscle fusion medium (see Recipes)
Equipment
- Cell culture incubator
- Fluorescence microscope
Procedure
- Skeletal myogenic priming
- For cells not natively committed to skeletal myogenesis (for example, cells isolated from non-muscle organs), a priming step may facilitate myogenic differentiation in vitro.
- To prime cells of interest for skeletal myogenesis, cells are plated on collagen type-I coated plates at low density (2 x 103 cells/cm2) for 5-7 days with muscle proliferation medium.
- Medium should be completely changed every 2 days.
- To further increase their myogenic efficiency, passage cells by typical trypsinization for 5 min at 37 °C and then repeat the priming step one or more times.
- For cells not natively committed to skeletal myogenesis (for example, cells isolated from non-muscle organs), a priming step may facilitate myogenic differentiation in vitro.
- Skeletal myogenic differentiation
- For human cells, cells are plated on collagen type-I coated 24- or 48-well plates at high confluency (>2.5 x 104 cells/cm2) for 7-14 days with human muscle fusion (myogenic) medium: DMEM high-glucose supplemented with 1% FBS, 1% HS, 0.5% CEE, and 1% P/S.
- Half of the fusion medium is renewed every 2-3 days until elongated, multinucleated skeletal myofibers appear (Crisan et al., 2008).
- For mouse cells, cells near or at confluency (>1.5 x 104 cells/cm2) are cultured for 3-7 days in mouse muscle fusion (myogenic) medium: DMEM high-glucose supplemented with 2% FBS and 1% P/S.
- Half of the fusion medium is renewed every 2 days until elongated, multinucleated skeletal myofibers appear (Lu et al., 2014).
- For human and mouse cells not tolerating sudden switch to low-serum fusion media with resultant cell death, skeletal myogenesis can be induced by gradually lowering serum concentration from 20% to 2% (for example, 20%-10%-5%-2%, each stage for 3-7 days) until elongated, multinucleated skeletal myofibers appear.
- A positive myogenic cell control (e.g., skeletal myoblast) can be included in the experiment with cells of interest to confirm the efficacy of myogenic media.
- For human cells, cells are plated on collagen type-I coated 24- or 48-well plates at high confluency (>2.5 x 104 cells/cm2) for 7-14 days with human muscle fusion (myogenic) medium: DMEM high-glucose supplemented with 1% FBS, 1% HS, 0.5% CEE, and 1% P/S.
- Skeletal myofiber detection
- Skeletal myogenic differentiation can be first identified by the distinct morphology of elongated, multinucleated skeletal myofibers (Figure 1).
- To precisely determine the myogenic differentiation, immunofluorescent staining for muscle cell markers: desmin (early myogenesis), fast- and slow-myosin heavy chain (fast- and slow-MyHC; mature myogenesis) is performed.
- For human cells, 7-14 days after culturing in muscle fusion medium, cells are rinsed two times with DPBS and then fixed for 5 min in cold methanol/acetone mixture (1:1, -20 °C) (Chen et al., 2015).
- For mouse cells, 3-7 days after culturing in muscle fusion medium, cells are rinsed two times with DPBS and then fixed for 5 min in cold methanol (-20 °C) (Lu et al., 2014).
- Alternatively, cells can be fixed with 10% formalin for 8 min at room temperature (RT) (Sohn et al., 2015).
- After washing 3 times with DPBS for 5 min each, fixed cells are blocked with 10% normal donkey serum for 1-2 h and then incubated, without washing, with the primary mouse anti-desmin, mouse anti-fast-MyHC, or mouse anti-slow-MyHC antibody (diluted in 5% normal donkey serum) for 2 h at RT or at 4 °C overnight.
- After washing 3 times with DPBS for 5 min each, the cells are then incubated with the secondary Alexa 594-conjugated anti-mouse IgG antibody or Alexa 488-conjugated anti-mouse IgG antibody (diluted with 5% normal donkey serum) for 30 min to 1 h at RT.
- After washing 3 times with DPBS for 5 min each, nuclei are stained by DAPI for 5 min.
- After washing 2 times with DPBS for 5 min each, stained cells can be observed using a fluorescent microscope.
- The percentage of differentiated myotubes can be quantified as the number of nuclei in MyHC-positive myotubes relative to the total number of nuclei.
- Stained cells can be preserved in sterile DPBS at 4 °C in the dark for up to 1 week.
- If staining background persists after washing, the primary antibody can be detected using a mouse-on-mouse (M.O.M) staining kit to reduce signal noise, according to the manufacturer's directions.
- Skeletal myogenic differentiation can be first identified by the distinct morphology of elongated, multinucleated skeletal myofibers (Figure 1).
Representative data
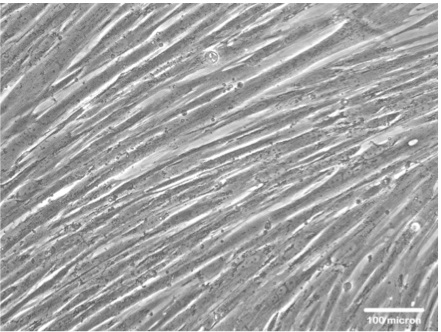
Figure 1. Representative data. A representative picture of human multi-nuclei skeletal myotube formation after myogenic induction for 7 days.
Notes
- We observed variability in skeletal myogenesis between early- and late-passage cells. Early-passage cells had higher skeletal myogenesis (with or without priming) in general.
- This protocol is not suitable for cells that could not proliferate to near confluency in high-glucose DMEM with high serum concentration.
Recipes
- Muscle proliferation medium
DMEM high-glucose supplemented with 10% fetal bovine serum (FBS), 10% horse serum (HS), 1% chicken embryo extract (CEE), and 1% Penicillin-Streptomycin (P/S) before switching to muscle fusion medium (Chen et al., 2015) - Muscle fusion medium
Human muscle fusion medium: DMEM high-glucose supplemented with 1% FBS, 1% HS, 0.5% CEE, and 1% P/S (Chen et al., 2015)
Mouse muscle fusion medium: DMEM high-glucose supplemented with 2% FBS and 1% P/S (Lu et al., 2014)
Acknowledgments
This protocol was adapted with modification from Chen et al. (2015). This work was supported by grants from the Medical Research Council (to B. P.), British Heart Foundation (to B. P.), National Institute of Health (R21HL083057 to B. P.).
References
- Chen, W. C., Baily, J. E., Corselli, M., Diaz, M. E., Sun, B., Xiang, G., Gray, G. A., Huard, J. and Péault, B. (2015). Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells 33(2): 557-573.
- Chen, W. C., Saparov, A., Corselli, M., Crisan, M., Zheng, B., Péault, B. and Huard, J. (2014). Isolation of blood-vessel-derived multipotent precursors from human skeletal muscle. J Vis Exp(90): e51195.
- Crisan, M., Yap, S., Casteilla, L., Chen, C. W., Corselli, M., Park, T. S., Andriolo, G., Sun, B., Zheng, B., Zhang, L., Norotte, C., Teng, P. N., Traas, J., Schugar, R., Deasy, B. M., Badylak, S., Buhring, H. J., Giacobino, J. P., Lazzari, L., Huard, J. and Péault, B. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3): 301-313.
- Gharaibeh, B., Lu, A., Tebbets, J., Zheng, B., Feduska, J., Crisan, M., Péault, B., Cummins, J. and Huard, J. (2008). Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc 3(9): 1501-1509.
- Lu, A., Poddar, M., Tang, Y., Proto, J. D., Sohn, J., Mu, X., Oyster, N., Wang, B. and Huard, J. (2014). Rapid depletion of muscle progenitor cells in dystrophic mdx/utrophin-/- mice. Hum Mol Genet 23(18): 4786-4800.
- Sohn, J., Lu, A., Tang, Y., Wang, B. and Huard, J. (2015). Activation of non-myogenic mesenchymal stem cells during the disease progression in dystrophic dystrophin/utrophin knockout mice. Hum Mol Genet 24(13): 3814-3829.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chen, W. C. W. and Péault, B. (2015). Skeletal Myogenesis in vitro. Bio-protocol 5(21): e1645. DOI: 10.21769/BioProtoc.1645.
Category
Stem Cell > Adult stem cell > Maintenance and differentiation
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









