- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Electroretinogram (ERG) Recordings from Drosophila
(*contributed equally to this work) Published: Vol 5, Iss 21, Nov 5, 2015 DOI: 10.21769/BioProtoc.1636 Views: 16115
Reviewed by: Oneil G. BhalalaMasahiro MoritaFabiana Scornik
Abstract
Phototransduction is a process in which light is converted into electrical signals used by the central nervous system. Invertebrate phototransduction is a process mediated by the phosphoinositide signaling cascade, characterized by Phospholipase C (PLC) as the effector enzyme and the Transient Receptor Potential (TRP) channels as its target. The great advantage of using invertebrate photoreceptors is the simplicity of the preparation, the ease of light stimulation, the robust expression of key molecular components, and most importantly, the ability to apply the power of molecular genetics. This last feature is mainly attributed to Drosophila melanogaster as a preferred animal model.
The Electroretinogram (ERG) is an extracellular voltage recording from the entire eye, which reflects the total electrical activity arising from the retina in response to a light stimulation. The Drosophila ERG light response is robust and easily obtained, thus making it a convenient method to identify defects in the light response as a result of mutations. The Prolonged Depolarizing Afterpotential (PDA) is a useful ERG phenomenon that can be recorded from white-eyed flies following intense blue light. It is induced by a massive photo-conversion of the photopigment rhodopsin to its dark stable state called metarhodopsin, due to failure of light response termination. Unlike the light coincident ERG recording, which declines quickly to the dark baseline after the cessation of the light stimulus, the PDA response continues long (hours) after light offset. However, this response can be suppressed to the dark baseline at any time by photo-conversion of metarhodopsin back to rhodopsin, by application of an intense orange light stimulus (see Figure 7; Minke, 2012). The PDA has been used as an important tool to screen for visual defective mutant (Minke, 2012).
Materials and Reagents
- Sterile disposable filter (0.2 μm pore size, aPES membrane, 75 mm diameter) [such as NalgeneTM Rapid-FlowTM Sterile Disposable Filter Units (Thermo Fisher Scientific)]
- 1 borosilicate glass capillary (OD 1.0 mm, ID 0.58 mm) (Harvard Apparatus)
- 3 silver filaments (1 mm diameter, 10 cm length) [such as Electrode AG/AGCL 1 mm DIA (WPI)]
- 1 ml/2 ml Syringe + 1 ml/2 ml Syringe with elongated tip [such as MEDI-PLUS 1ml without needle (KDL Medical Product Company)]
- Syringe filter (0.22 μm pore size, suitable for a 4 mm syringe tip) with PVDF membrane [such as Millex-GV Syringe Driven Filter Unit (Merck Millipore Corporation)]
- White-eyed Drosophila flies (available for purchase at Bloomington Drosophila Stock Center)
Note: Red-eyed Drosophila flies are also suitable for ERG recordings, but a PDA cannot be induced in these flies. - Homemade low temperature melting wax composed of mixture of paraffin and bee wax
- Redux cream for electrocardiography [such as Redux Electrolyte Crème (Parker Laboratories)]
- Ringer’s solution (see Recipes)
Equipment
- Horizontal Micropipette puller [such as programmable Flaming/Brown type micropipette puller (Sutter Instrument Company, model: P-97 )] with platinum filament. Less expensive pullers [such as Narishige vertical puller (NARISHIGE Group, model: PP-830 )] can also be used
- Pulsed gas flow anesthesia system with injector [such as the Sleeper system (Inject+Matic Sleeper)] connected to CO2 tank
- Fly stand [such as Alnico Shallow Pot Magnet (Eclipse Magnetics)] (Figure 1, #2, Figure 2, #2 and Video 1)
- Fine tweezers [such as tweezers (Biologie, model: number 5 )] and fine paint brush [such as paint brush (Rekab, model: series 40, number 3 )]
- Homemade Wax filament heater + soldering iron composed of a platinum-iridium (0.25 mm diameter) filament and a holder (Figure 3)
- Dissecting Stereoscopic zoom Microscope [such as Nikon Stereozoom Microscope (Nikon Corporation, model: SMZ-2 )]
- “Cold” illuminator [such as KL 1500 LCD (SCHOTT North America, model: KL 1500 LCD )] with heat and red filters [such as KG3 and RG620, respectively (SCHOTT North America, models: KG3 and RG620 )]
- Computer with electric signal processor (such as Clampex) and data analysis program (such as Clampfit)
- Stereo-microscope with at least 4 different magnification settings [such as the Wild M5 (Leica Microsystems) with 6, 12, 25 and 50 magnification settings] (see Figure 1, #3 and Video 1)
- Vibration Isolated Table
- Dark Faraday cage covered in black cloth (Figure 1, #6 and Video 1).
- On/Off magnet block to provide convenient placement of the fly stand [such as complete Magnetic Base (Eclipse Magnetics, model: E905 )] (Figure 2, #5 and Video 1)
- 4 triple axis coarse mechanical micromanipulators [such as the Three-Axis Compact Coarse Micromanipulator (Tritech Research, Narishige, model: M-2 )] (Figure 1, #4, Figure 2, #3 and Video 1)
- 1 triple axis, fine, very stable mechanical micromanipulator for holding the recording electrode [such as the Leitz Mechanical Micromanipulator (Leica Microsystems)] (Figure 1, #8)
- Light detector phototransistor for light monitoring (to be fixed on one of the micromanipulators) (Figure 1, #5, Figure 2, #4 and Video 1).
- Shutter system [such as LS2 2 mm Uni-stable Shutters (Uniblitz, Vincent Associates®)] + shutter driver [such as VCM-D1 Single Channel Uni-stable (Uniblitz, Vincent Associates®)]
- High-pressure ozone-free 75 W Xenon lamp (operating on 50 W) + Schott KG3 heat filter + 2 condenser lenses + fiber optic (3 mm diameter, 1.3 m long) conducting light into the Faraday cage for light stimulation
- Lamp power supply (such as PTI LPS-220)
- Data acquisition system [such as Digidata 1550 digitizer (Molecular Devices, model: Digidata 1550 digitizer )]
- 2 glass electrode holders suitable for capillary O.D. 1 mm (Figure 1, #1, Figure 2, #1 and Video 1)
- Microelectrode preamplifier system with head-stage impedance tester, at least x10 amplification [such as Extracellular Preamplifier Current Pump System (Dagan, model: 2400A )] (Figure 1, #7 and Video 1)
- Pulse generator [such as Master 8 and Master 9 (A.M.P.I, models: Master 8 and Master 9 )]
- Neutral Density (ND) filters [such as ND filters (Balzer) and color filters including Red (SCHOTT North America, model: RG610 ), Orange (SCHOTT North America, model: OG590 ) and Blue (SCHOTT North America, model: BG63 )]
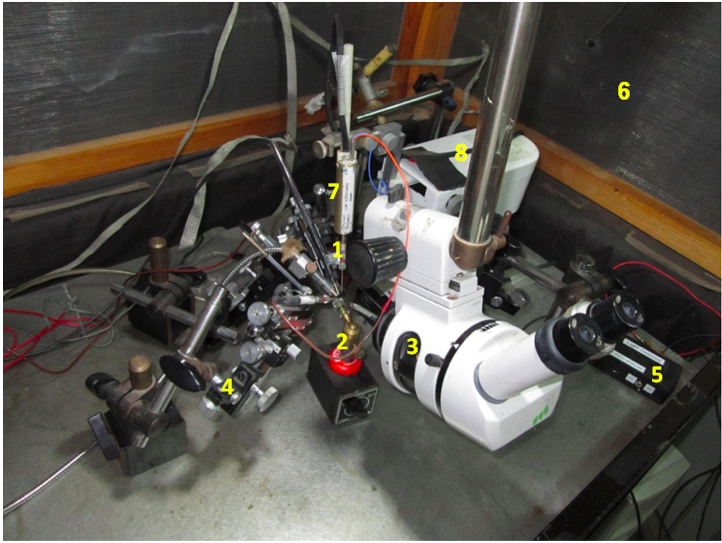
Figure 1. An overview of the ERG setup. #1-electrode holder, #2-fly stand, #3- stereo-microscope, #4-micromanipulator, #5-light detector phototransistor, #6- Faraday cage, #7-amplifier head-stage, #8-Leica-Leitz Mechanical Micromanipulator.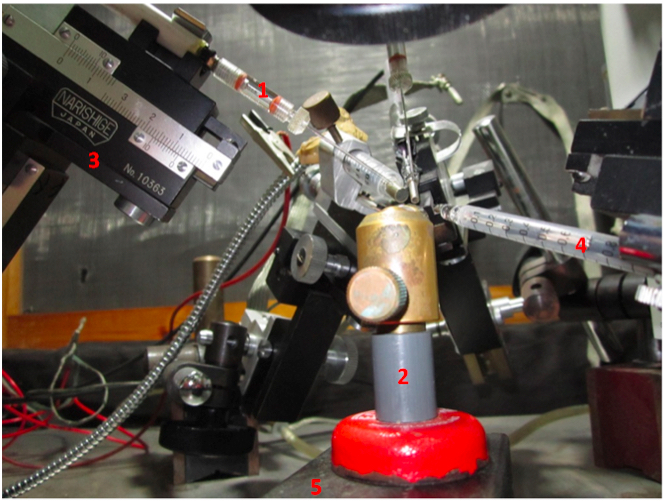
Figure 2. A magnified view of the ERG setup. #1-electrode holder, #2-fly stand, #3- micromanipulator, #4-light detector probe, #5-magnet block.
Figure 3. A homemade instrument for immobilizing the living fly. A platinum-iridium (0.25 mm diameter) filament is electrically connected to a power supply with a controlled current output. The filament is attached via insolating plastic to a copper rod holder (heater and soldering iron).
Video 1. A general overview of the ERG set
Procedure
The main components of the light response recorded by the ERG are (1) the extracellularly recorded photoreceptor potential, (2) the “on” and “off” transients, at the beginning and the end of the light pulse, arising from the second order lamina neurons, and (3) the slow response of the pigment (glia) cells. The photoreceptor potential is the physiological response to light arising from the light-induced openings of the Transient Receptor Potential (TRP) and TRP-Like (TRPL) channels. In response to intense step of light, the photoreceptor’s component of the ERG is composed of an initial corneal negative fast transient, which declines to a lower steady state phase due to light adaptation. The depolarization of the photoreceptor cell triggers the induction of the corneal positive “on” transient, via sign inverting synapse between the photoreceptor axon and the large monopolar neurons of the lamina. The response of the pigment cells appears as a corneal negative slow rise and slow decay of the ERG after light on and off, respectively. These slow components arise from an increase in extracellular K+, resulting from K+ efflux from the photoreceptor cells via the TRP and TRPL channels, which depolarize the pigment cells surrounding the photoreceptor cells (see Figure 6; Minke, 1982).
- Preparing electrodes.
- Insert each of the two silver-chloride filaments into the electrode holders, ensuring that the end of the filament makes contact with the metal bottom of the holder.
- Carefully insert glass capillary into micropipette puller, and set puller program to create pipettes with adequate tip size (approximately 1 µm), taper (approximately 4 mm) shape and resistance of 5-10 MΩ. These parameters may be achieved by using three heating cycles on the programmable P-97 Flaming/Brown puller (note that these settings are not required in cheaper pullers).
- Load the elongated tip syringe with Ringer's solution, using an additional syringe, filtering the solution through a syringe filter as you do so. Fill each glass capillary with Ringer's solution, using the elongated syringe (ensure that the capillary is at least half full).
- Slide the silver-chloride filament (attached to the electrode holder) into the glass capillary.
- Insert one electrode holder with the recording pipette into the electrode micromanipulator that will be used for grounding (ground electrode, as shown in Figure 2, #1), and insert the other electrode holder with the recording pipette into the preamplifier head-stage (active electrode, as shown in Figure 1, #7 and Video 1).
- Insert each of the two silver-chloride filaments into the electrode holders, ensuring that the end of the filament makes contact with the metal bottom of the holder.
- Ensure that the work-set is properly connected and turned on (as shown in Figure 1, Figure 3 and Video 1).
- Preparing a live fly for the experiment: All the below procedure should be done under deep red illumination to keep the fly under dark adapted conditions. To obtain deep red illumination insert red filters into the “Cold” illuminator and into the Xenon lamp.
- Switch on the "Cold" illuminator to provide red light during the fixation of the fly.
- Anesthetize flies in the vial using the fly sleeper injector.
- Pour flies out of the vial, on to the sleeper container, while pressing down on the CO2 emitting pedal.
- Choose one fly for the experiment, and cover the rest with a Petri dish.
- Ensure the fly is in the proper orientation-lying on its side, with its back towards the hand that will be used to fix the fly on the fly holder (as shown in Figure 4).
- Place a drop of wax on the soldering iron.
- Lift the fly from its wings with the tweezers, and use the soldering iron to fix its wings to the fly stand (see Figure 4).
- Using the soldering iron and wax drops, fix the following parts of the fly (see Figure 4):
- The back: Connect most of the fly’s back to the stand surface with wax.
- The legs: Lower the tip of the soldering iron onto the joining point of the legs, wait as the fly brings its legs to that point, and melt the wax to cover all the legs together.
*Take care not to cover the thorax and abdominal sides of the fly, where the fly trachea openings are located, for that will prevent it from breathing. - The head: carefully place a drop of low temperature melting wax between the head and the back, in the neck area.
*Take care not to overheat the head but to cover the upward facing eye with wax as you fix the head to the back. Overheating the head would not kill the fly but would eliminate the light response. - The trunk-optional: Using a small drop of wax, connect the trunk to the upper end of the torso.
*Ensure that the fly head, thorax and legs are properly fixed, and are unable to move during the experiment.
*Even small movements of these organs would cause large electrical artifacts.

Figure 4. A living fly properly fixed to the fly stand with wax - The back: Connect most of the fly’s back to the stand surface with wax.
- Place a drop of Redux cream on the lower end of the fly's body.
- Place the stand in the Faraday cage, on the magnet block (when in the ON mode), and ensure that the fly is approximately 0.5 cm from the end of the light guide.
- Look through the smallest amplification of the stereo-microscope to roughly position the recording electrode above the fly's eye, and the ground electrode over the fly's upper torso/upper back, using the micromanipulators.
- Gradually enlarge the magnification of the stereo-microscope (usually x 25 is sufficient magnification), ensuring that the fly is still in the center of the field of view (as shown in Figure 5).
- Insert the ground electrode into the upper torso/back of the fly, using the micromanipulator (as shown in Figure 5).
- Preferably, insert the recording active electrode into the outer periphery of the fly's eye (the area where the tissue is a bit sturdier), using the micromanipulator.
*The ERG amplitude is roughly similar at all corneal regions. Therefore, the exact location of the recording electrode on the cornea is not critical.
Figure 5. Fly with electrodes placed in the eye and torso at high (left) and low (right) magnifications
- Switch on the "Cold" illuminator to provide red light during the fixation of the fly.
- Close the Faraday cage, ensuring it is completely dark.
*It is preferable to adjust the amplifier DC offset to 0 before performing the recordings, but it is not critical since the experimental objective is to measure the ERG amplitude relative to the dark baseline.
*Ensure good electrical connections between all metal parts of the setup and ground. This is important to eliminate pickup of the 50/60 Hz of the lab electricity. - ERG Intensity Response protocol:
- Start documenting the experiment, using the electric signal processor computer program.
- Place an orange filter in front of the Xenon lamp, together with an ND -log6 filter.
- Wait at least 30 sec in the dark, and then apply a 5 sec light pulse, using the pulse generator.
- Replace the ND -log6 filter with an ND -log5 filter (or any other ND log filter, as required).
- Wait at least 30 sec in the dark, and then apply an additional 5 sec light pulse, using the pulse generator.
- Repeat steps 5d-e, while you gradually increase the light intensity using the other ND log filters (the last pulse should be generated with no filter at all).
*It is important to begin with dim lights and gradually increase the light intensity since this would keep the fly in a relative dark adapted state suitable for the subsequent more intense stimulus.
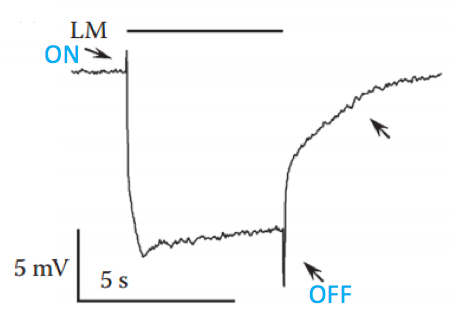
Figure 6. Typical ERG trace recorded from wild type fly in response to intense orange light. The upper filled bar represents the light monitor (LM); the down-pointing upper arrow indicates the “on” transient and the bottom up-pointing arrow indicates the “off” transient, both arising from the second order lamina neurons; the right up-pointing arrow indicates a slow component arising from the pigment (glia) cells; the slowly rising corneal negative peak amplitude also originates from the pigment cells. - Start documenting the experiment, using the electric signal processor computer program.
- PDA protocol:
- Use white-eyed fly; place an orange filter in front of the Xenon lamp and apply two/three 5 sec light pulses, at 5-10 sec dark intervals using the pulse generator.
- Wait at least 30 sec in the dark, place a blue filter in front of the Xenon lamp, and apply two/three 5 sec light pulses, at 5-10 sec dark intervals.
- Wait at least 30 sec in the dark, place an orange filter in front of the Xenon lamp, and apply two 5 sec light pulses, at 5-10 dark intervals.
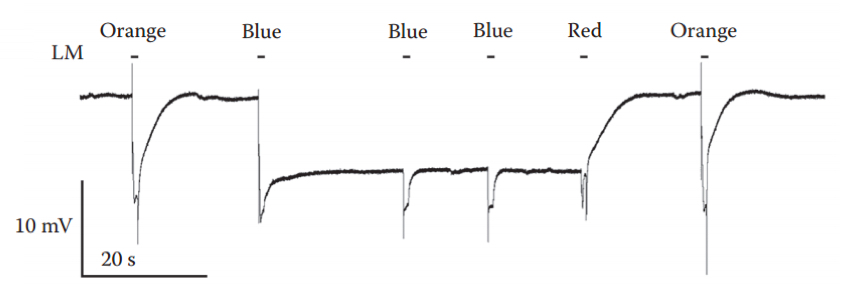
Figure 7. Induction and suppression of a PDA. An intense blue light pulse, which converted ~80% of the rhodopsin photopigment to its metarhodopsin state resulted in a saturated prolonged corneal negative response that continued in the dark, owing to maximal activation of the TRP and TRPL channels, making R1-6 cells non responsive. Two additional intense blue lights elicited smaller responses (without the ON and OFF transients) that originated from the R7 and R8 cells, in which a PDA was not induced. The following red light, which photoconverted metarhodopsin back to rhodopsin suppressed the PDA after the light was turned off. Intense orange light pulses were applied before and after the PDA. LM: light monitor. - Use white-eyed fly; place an orange filter in front of the Xenon lamp and apply two/three 5 sec light pulses, at 5-10 sec dark intervals using the pulse generator.
- Analyze traces using a data analysis program (see analysis example in Figure 8).
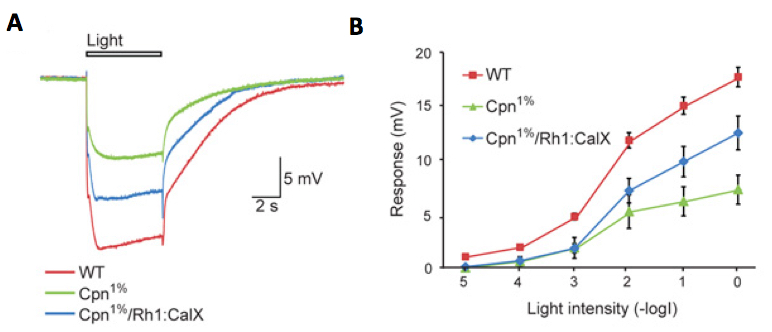
Figure 8. An example of ERG intensity response analysis of Cpn1% Drosophila strain. Cpn1% strain is a Calphotin-RNAi induced transgenic fly, in which the endogenous Ca2+ buffer protein, calphotin (CPN) is reduced to 1% of normal, in R1-6 photoreceptors (Weiss et al., 2012). A comparison of ERG responses to orange light pulses among three fly strains: A. Superposition of 3 sample traces showing the ERG responses to maximal intensity orange light pulses of wild type (WT, control), Cpn1% and Cpn1%/Rh1:CalX rescue flies (transgenic Cpn1% flies, which also over-express a Ca2+ pump that extrudes Ca2+ from the photoreceptor cell). B. Intensity-response curves of ERG responses of the above three fly strains. The different curves were measured from WT, Cpn1% and Cpn1%/Rh1:CalX flies (mean ± S.E.M, n=4-8).
Notes
- Raising flies: Standard Drosophila protocol dictates that flies are raised on a standard corn starch substrate, supplemented with yeast extracts and at 24 °C. It is recommended that the flies used in the experiments do not grow in an over-populated environment.
For intensity response and PDA protocols, use flies that were raised in the dark, for at least 24 h (preferably 48-72 h). In addition, all steps of the fly preparation must be performed in the dark, using red lights only.
For optimal ERG recording, use flies 1-3 days old. - In cases where it is important to maintain the integrity of the eye, it is possible to place a dot of Redux cream on the top of the eye, and insert the electrode into the cream only, without piercing the eye.
Recipes
- Ringer's solution
Mix the ingredients according to the order of their appearance in the table aboveIngredient Con. mM NaCl 130 KCl 2 MgCl2 5 CaCl2 2 HEPES (pKa=7.5 @ 25 °C) 10
Adjust pH to 7.15, using HCl
Filter through a sterile disposable filter, and stored at 4 °C (Indefinite shelf life if stored properly)
Acknowledgments
We thank Anatoly Shapochnikov for constructing the Wax Filament Heater. The construction of the ERG protocol was supported by the Israel Science Foundation (ISF).
References
- Kohn, E. and Minke, B. (2011). Methods for studying Drosophila TRP channels. TRP Channels. M. X. Zhu. Boca Raton (FL).
- Minke, B. (1982). Light-induced reduction in excitation efficiency in the trp mutant of Drosophila. J Gen Physiol 79(3): 361-385.
- Minke, B. (2012). The history of the prolonged depolarizing afterpotential (PDA) and its role in genetic dissection of Drosophila phototransduction. J Neurogenet 26(2): 106-117.
- Weiss, S., Kohn, E., Dadon, D., Katz, B., Peters, M., Lebendiker, M., Kosloff, M., Colley, N. J. and Minke, B. (2012). Compartmentalization and Ca2+ buffering are essential for prevention of light-induced retinal degeneration. J Neurosci 32(42): 14696-14708.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Rhodes-Mordov, E., Samra, H. and Minke, B. (2015). Electroretinogram (ERG) Recordings from Drosophila. Bio-protocol 5(21): e1636. DOI: 10.21769/BioProtoc.1636.
-
Weiss, S., Kohn, E., Dadon, D., Katz, B., Peters, M., Lebendiker, M., Kosloff, M., Colley, N. J. and Minke, B. (2012). Compartmentalization and Ca2+ buffering are essential for prevention of light-induced retinal degeneration. J Neurosci 32(42): 14696-14708.
Category
Neuroscience > Neuroanatomy and circuitry
Neuroscience > Sensory and motor systems
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link