- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Vacuole Structure Analysis during Cell Death Subsequent to Application of Erwinia carotovora Culture Filtrates to Cell Cultures of Nicotiana tabacum
Published: Vol 5, Iss 20, Oct 20, 2015 DOI: 10.21769/BioProtoc.1629 Views: 8446
Reviewed by: Samik BhattacharyaDaniel SavatinAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A β-glucuronidase (GUS) Based Bacterial Competition Assay to Assess Fine Differences in Fitness during Plant Infection
Julien S. Luneau [...] Alice Boulanger
Jul 5, 2022 3222 Views

Tomato Stem Injection for the Precise Assessment of Ralstonia solanacearum Fitness in Planta
Yaru Wang [...] Alberto P. Macho
Aug 20, 2021 3726 Views

Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2775 Views
Abstract
We recently established an experimental model system for efficient defense-related cell death using tobacco BY-2 cultured cells treated with culture filtrates of the pathogenic bacterium Erwinia carotovora (E. carotovora) (Hirakawa et al., 2015). Applying this experimental system to transgenic BY-2 cells stably expressing the vacuolar membrane marker GFP-VAM3 (Kutsuna and Hasezawa, 2002) allowed us to monitor changes in vacuolar membrane structures including a decrease of transvacuolar strands during cell death (Hirakawa et al., 2015). Our model system can help to investigate organelle dynamics in defense-related cell death. Here, we show protocol for applying E. carotovora filtrates to BY-2 cells and confocal observation of vacuolar membrane dynamics and subsequent cell death. We used cell cycle synchronized BY-2 cells to effectively monitor invaginated vacuolar membranes such as transvacuolar strands in our recent report (Hirakawa et al., 2015); however, we do not describe the protocol for cell cycle synchronization in this article. For the step-by-step protocol for BY-2 cell synchronization, please refer to previous protocol papers (Nagata and Kumagai, 1999; Kumagai-Sano et al., 2006).
Keywords: Cell deathMaterials and Reagents
- Sterile filter with pore size 0.22 µm (Merck Millipore Corporation, Millex®–GV Filter unit, catalog number: SLGV033RS )
- 50 ml syringe (TERUMO CORPORATION, catalog number: SS-50ESZ )
- 12 well plate (Sumitomo Bakelite Co., catalog number: MS-80120 )
- Erwinia carotovora subsp. carotovora (National Institute of Technology and Evaluation, catalog number: 103133)
- Transgenic tobacco BY-2 cell culture (N. tabacum L. cv. Bright Yellow 2) stably expressing GFP-VAM3 (RIKEN Bioresource Center, catalog number: RPC00039 )
- Aphidicolin (Wako Pure Chemical Industries, Siyaku, catalog number: 015-09814 )
- Yeast extract (KANTO KAGAKU, KANTO Chemical, catalog number: 712021-5 )
- BactoTM Tryptone (BD bioscience, catalog number: 211705 )
- NaCl (KANTO KAGAKU, KANTO Chemical, catalog number: 37144-01 )
- Murashige and Skoog plant salt mixture (Wako Pure Chemical Industries, Siyaku, catalog number: 392-00591 )
- Sucrose (Wako Pure Chemical Industries, Siyaku, catalog number: 196-00015 )
- myo-Inositol solution (Wako Pure Chemical Industries, Siyaku, catalog number: 094-00281 )
- Thiamine hydrochloride (Wako Pure Chemical Industries, Siyaku, catalog number: 201-00852 )
- Sodium 2,4-Dichlorophenoxyacetate Monohydrate (Tokyo Chemical Industry, catalog number: D1319 )
- Lysogeny broth (LB) medium (see Recipes)
- Modified Linsmaier and Skoog (LS) medium (see Recipes)
Equipment
- Rotary shaker for E. carotovora culture (TAITEC CORPORATION, model: BR-40LF )
- 300 ml flask for E. carotovora culture (Sansyo, Iwaki, catalog number: 82-0087 )
- Spectrophotometer (Beckman Coulter, model: DU® 640 )
- Centrifuge (TOMY SEIKO CO, model: MX-300 )
- Deep freezer (Nihon Freezer, catalog number: CLN-30U )
- 100 ml flask for BY-2 culture (Sansyo, Iwaki, catalog number: 82-0085 )
- Rotary shaker for the untreated BY-2 culture (TAITEC CORPORATION, catalog number: 300LF )
- Rotary shaker for the treated BY-2 culture (TAITEC CORPORATION, catalog number: NR-30 )
- Incubator for the treated BY-2 culture (Sanyo, catalog number: MIR-553 )
- Glass base dish (Sansyo, Iwaki, catalog number: 3911-035 )
- Confocal laser scanning microscope (OLYMPUS, model: FV300 )
Procedure
- Preparation of E. carotovora culture filtrate
- Culture 100 ml of E. carotovora in liquid LB medium in a 300-ml flask at 37 ℃ overnight with rotary shaking at 150 rpm.
- Check OD600 with a spectrophotometer. The OD600 value should be in the range 0.8-1.0 for appreciable cell death induction.
- Centrifuge the bacterial cell culture for 10 min at 4,000 x g at room temperature.
- Collect the supernatant and sterile filter it with a syringe filter.
- The filtrate can be kept at -80 ℃ in a deep freezer for several months.
- Culture 100 ml of E. carotovora in liquid LB medium in a 300-ml flask at 37 ℃ overnight with rotary shaking at 150 rpm.
- Application of the filtrate to cell cycle synchronized tobacco culture cells
- Prepare the S-phase synchronized transgenic BY-2 cells expressing GFP-VAM3 by DNA polymerase inhibitor (aphidicolin) treatment and wash out with fresh modified LS medium (Nagata and Kumagai, 1999; Kumagai-Sano et al., 2006).
- Just after the washout, place 30 ml of the synchronized culture cells in a 100-ml flask on a rotary shaker culture at 27 ℃ for 30 min at 130 rpm.
- Add 1.6 ml of the cell cycle synchronized culture into a well of the 12-well plate.
- Add 0.4 ml of the E. carotovora culture filtrate into the cell culture well [final concentration: 20% (v/v)].
- Culture the treated cells on a rotary shaker in an incubator at 27 ℃ for two hours.
- Collect 150 μl of the cells into a microscope glass dish base for observation.
- Place the dish on the stage of the confocal laser scanning microscope.
- Acquire time-lapse images of GFP-VAM3 at 5-min intervals with 488 nm excitation lasers and 524-546 nm emission filters according to the microscope manufacturer’s instructions. See Figure 1 for representative results.
- Prepare the S-phase synchronized transgenic BY-2 cells expressing GFP-VAM3 by DNA polymerase inhibitor (aphidicolin) treatment and wash out with fresh modified LS medium (Nagata and Kumagai, 1999; Kumagai-Sano et al., 2006).
Representative data
The representative time-lapse images of GFP-VAM3 were shown in Figure 1. After the filtrate treatment, the invaginated vacuolar membrane structures including transvacuolar strands gradually decreased (Figure 1). The percentage of transvacuolar strand-less cells reached 12.8±3.00% 4 h after the filtrate treatment while 1.52±1.48% in the mock treatment (Hirakawa et al., 2015).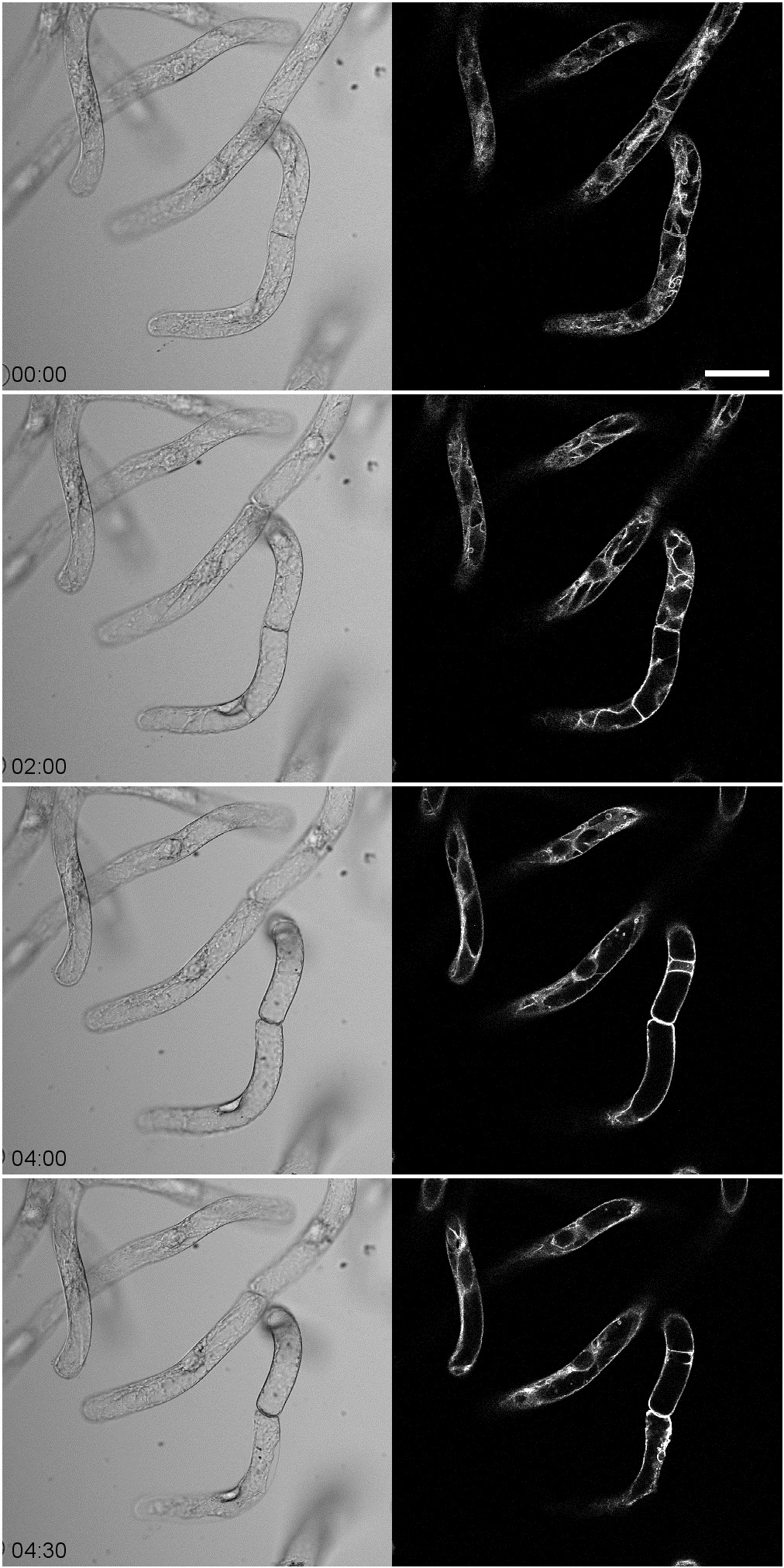
Figure 1. Time-lapse observation of tobacco BY-2 cells expressing GFP-VAM3 treated with culture filtrates of Erwinia carotovora. Note that the invaginated vacuolar membranes gradually decreased before cell death with cell shrinkage. The time-lapse images were obtained at 5-min intervals for 4.5 h. Scale bar indicates 50 μm.
Notes
You should wait around 10 min after the placement of a microscope glass dish base on the microscope stage to start a time-lapse imaging. The cells would be completely settled down to the bottom while waiting, resulting in appreciated results without the cell displacement.
Recipes
- LB medium
Note: No need to adjust pH.Yeast extract 5 g/L BactoTM tryptone 10 g/L NaCl 5 g/L - Modified LS medium
Murashige and Skoog plant salt mixture 4.6 g/L Sucrose 30 g/L myo-Inositol solution 200 mg/L Thiamine hydrochloride 1 mg/L 2,4-Dichlorophenoxyacetic acid 0.2 mg/L Adjust pH to 5.8 with KOH
Acknowledgments
The authors thank Dr. Toshihisa Nomura for preliminary experiments on E. carotovora culture filtrates. This work was supported by JSPS KAKENHI Grant Numbers 25711017 (T.H.), 25291056 (S.H.) and 24114007 (S.H.).
References
- Hirakawa, Y., Nomura, T., Hasezawa, S. and Higaki, T. (2015). Simplification of vacuole structure during plant cell death triggered by culture filtrates of Erwinia carotovora. J Integr Plant Biol 57(1): 127-135.
- Kumagai-Sano, F., Hayashi, T., Sano, T. and Hasezawa, S. (2006). Cell cycle synchronization of tobacco BY-2 cells. Nat Protoc 1(6): 2621-2627.
- Kutsuna, N. and Hasezawa, S. (2002). Dynamic organization of vacuolar and microtubule structures during cell cycle progression in synchronized tobacco BY-2 cells. Plant Cell Physiol 43(9): 965-973.
- Nagata, T. and Kumagai, F. (1999). Plant cell biology through the window of the highly synchronized tobacco BY-2 cell line. Methods Cell Sci 21(2-3): 123-127.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hirakawa, Y., Hasezawa, S. and Higaki, T. (2015). Vacuole Structure Analysis during Cell Death Subsequent to Application of Erwinia carotovora Culture Filtrates to Cell Cultures of Nicotiana tabacum. Bio-protocol 5(20): e1629. DOI: 10.21769/BioProtoc.1629.
Category
Plant Science > Plant immunity > Disease bioassay
Microbiology > Microbe-host interactions > Bacterium
Microbiology > Microbe-host interactions > Ex vivo model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












