- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Clonal Culture of Mouse Liver Progenitor Cells
Published: Vol 5, Iss 20, Oct 20, 2015 DOI: 10.21769/BioProtoc.1624 Views: 12660
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3941 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2413 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 233 Views
Abstract
Liver stem/progenitor cells (LPCs) are defined as bipotential cells differentiating into both hepatocytes and cholangiocytes. For analyzing their differentiation potential, clonal culture has been used for LPCs isolated by a cell sorter. In addition, we can use the culture to assess functions of target genes on differentiation potential of LPCs. This protocol describes the process of cell isolation and colony assay to examine proliferative and differentiation potential of LPCs.
Keywords: BipotentialMaterials and Reagents
- Autoclaved 250 μm Nylon mesh (Nippon Rikagaku Kikai)
- Butterfly needle (23 gauge) (Terumo, catalog number: SV-23CLK )
- FalconTM Cell Strainers (Falcon, catalog number: 332350 )
Note: Currently, it is “Thermo Fisher Scientific, Falcon™, catalog number: 332350 ”. - 35 mm tissue culture dish (Corning, catalog number: 430165 )
- Collagenase (used at 1 mg/ ml for perfusion) (Wako Pure Chemical Industries, catalog number: 032-10534 )
- PBS
- Hanks’ balanced salt solution (HBSS) (Sigma-Aldrich, catalog number: H9269 )
- Ethylene glycol-bis (2-aminoethylether)-N, N, N′, N′-tetraacetic acid (Sigma-Aldrich, catalog number: E0396 )
- Deoxyribonuclease I from bovine pancreas (Sigma-Aldrich, catalog number: DN25 )
- Hyaluronidase (Sigma-Aldrich, catalog number: H3566 )
- Laminin 111 (BD biosciences, catalog number: 354232 )
Note: Currently, it is “Corning, catalog number: 354232”. - Matrigel® Growth Factor Reduced (GFR) Basement Membrane Matrix, *LDEV-Free, 10 ml (BD biosciences, catalog number: 354230 )
Note: Currently, it is “Corning, catalog number: 354230”. - Recombinant Mouse Oncostatin M (OSM) Protein (R&D systems, catalog number: 495-MO )
- EGF Recombinant Human Protein (Life technologies, catalog number: PHG0311 )
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: PHG0311”. - Recombinant Human HGF (NS0-expressed) Protein (R&D systems, catalog number: 294-HGN )
- Insulin from bovine pancreas (Sigma-Aldrich, catalog number: I5500 )
- Insulin/Transferrin/Selenium (ITS) (Life Technologies, catalog number: 41400-045 )
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 41400-045”. - Dexamethasone (Dex) (Sigma-Aldrich, catalog number: D-4902 )
- Anti-CD16/32 antibody (Biolegend, catalog number: 101-301 )
- FITC anti-Dlk1 antibody (MBL International, catalog number: D187-4 )
- FITC anti-mouse CD326 (Ep-CAM) Antibody (Biolegend, catalog number: 118207 )
- APC-Cy7 anti-CD45 (Biolegend, catalog number: 103-115 )
- APC-Cy7-TER119 (Biolegend, catalog number: 116-223 )
- PE-Cy7-anti-CD31 (Biolegend, catalog number: 102417 )
- Paraformaldehyde (PFA)
- Hoechst 33258 (Dojindo Molecular Technologies, catalog number: 343-07961 )
- Anti-mouse albumin (ALB) antibody (Bethyl laboratories, catalog number: A90-134P )
- Anti-cytokeratin 19 (CK19) (Tanimizu et al., 2003)
- AlexaFluor 488 conjugated donkey anti-rabbit IgG (Life technologies, catalog number: A-21206 ) and AlexaFluor 555 conjugated donkey anti-goat IgG (Life Technologies, catalog number: A-21432)
Note: Currently, it is “Thermo Fisher Scientific, Novex™, catalog number: A-21206 and A-21432 ”. - Prolong Gold (Life Technologies, catalog number: P36930 )
Note: Currently, it is “Thermo Fisher Scientific, Molecular Probes™, catalog number: P36930”. - L-15 medium (Sigma-Aldrich, catalog number: L4386 )
- DMEM/nutrient mixture Ham F-21 (DMEM/F12) medium
- Decomplemented Hyclone fetal bovine serum (FBS) (Thermo Fisher Scientific, catalog number: SH30910.03 )
Note: Currently, it is “GE Healthcare, catalog number: SH30910.03”. - Niflumic acid (Sigma-Aldrich, catalog number: N0630 )
- Blockace (DS Pharma Biomedical Co, catalog number: UK-B40 )
- Cloning ring (ASAHI GLASS CO, catalog number: 11-016-006 )
- Trypsin/EDTA (Sigma-Aldrich, catalog number: T4049 )
- RNAiMAX (Life Technologies, catalog number: 13778030 )
Note: Currently, it is “Thermo Fisher Scientific, Invitrogen™, catalog number: 13778030”. - RNeasy Mini Kit (QIAGEN, catalog number: 74106 )
- ROCK inhibitor (Wako Pure Chemical Industries, catalog number: 257-00511 )
- Pre-perfusion solution (see Recipes)
- Perfusion solution (see Recipes)
- L-15 medium (see Recipes)
- Hyaluronidase (see Recipes)
- Culture medium (see Recipes)
Equipment
- Centrifuge (KUBOTA)
- FACSAriaII (BD biosciences)
- CO2 incubator (The incubator is used to keep culture at 37 °C and under 5% CO2) (SANYO)
- Fluorescence microscope (OLYMPUS, model: IX71 ) and digital camera (OLYMPUS, model: DP72 )
- Plate reader Mutiskan JX (Labsystems)
- ROCK inhibitor (Wako Pure Chemical Industries, catalog number: 257-00511 )
- Round-shaped stirring bar (ASONE Corporation, catalog number: 1-5409-01 )
Procedure
- Liver tissue is digested by two-step collagenase perfusion from the portal vein. A butterfly needle is inserted into the portal vein and then 25 ml of pre-perfusion solution is injected by using a peristaltic pump at 6 ml/min. During this step, 50 mg collagenase is added to 50 ml perfusion solution and quickly dissolved by gentle shaking. Then, the liver is perfused with the perfusion solution containing collagenase by using a peristaltic pump at 3 ml/min.
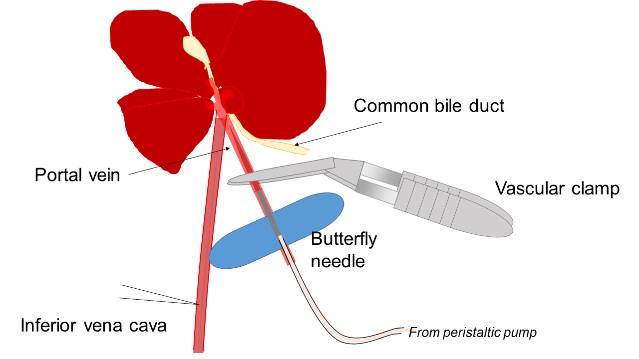
Figure 1. A schematic view of liver perfusion. After anesthesia, the abdomen is opened and the portal vein is identified by putting the gastrointestinal tract to the right side. A butterfly needle is inserted into the portal vein and fixed by a vascular clamp. The liver turns into pale color by starting perfusion and then cut the inferior vena cava to drain perfusion solution. - The liver is washed in HBSS to eliminate hepatocytes. Undigested tissue is incubated in 10 ml of L-15 medium containing 80 mg collagenase and 50 μl of 1 mg/ml DNase I with gentle stirring at 37 °C for 10 min. After passing through a nylon mesh, undigested tissue is cut into small pieces, resuspended in 25 ml of L-15 medium containing 100 mg collagenase, 50 μl of 1 mg/ml DNaseI, and 25 μl of hyaluronidase solution with vigorous stirring at 37 °C for 40 min. Cell suspension is passed through a nylon mesh and then through a 40 μm cell strainer. DMEM/F12 medium containing 10% FBS is added to cell suspension to stop enzymatic digestion. After eliminating cell clumps by centrifugation at 50 x g for 1 min, supernatant is centrifuged at 350 x g for 4 min to collect dissociated cells. It is expected to acquire about 5 x 106 cells from one mouse.
- After resuspending cells in 200 μl of DMEM/F12 medium, 2 μl of anti-CD16/32 (FcγIII/II receptor) antibody is added and incubated at 4 °C for 30 min to avoid non-specific binding of antibodies through the Fc region of immunoglobulins.
- Chilled PBS containing 1% FBS is added and cell suspension is centrifuged at 350 x g for 4 min.
- Cells are resuspended in 200 μl DMEM/F12 medium containing 10% serum and incubated with 1 μl of fluorescence dye-conjugated antibodies. Cells are incubated at 4 °C for 30 min. CD45-TER119-CD31-EpCAM+ cells are isolated by FACS (Figure 2).
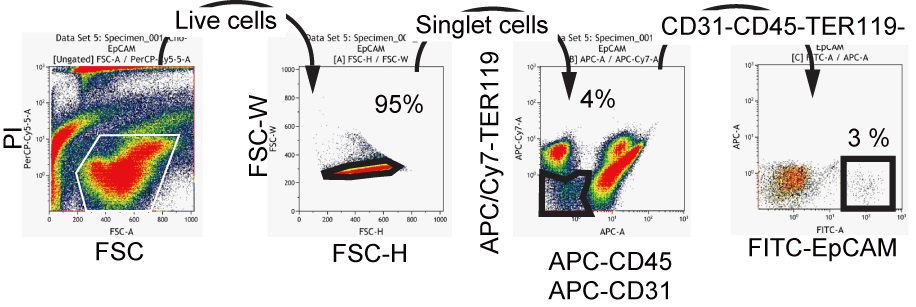
Figure 2. A typical FACS chart for EpCAM+ cells isolation. Live cells in the propidium iodide-negative (PI-) fraction are further gated to get singlet cells on FSC-H (height)/FSC-W (width) plot. Next, non-hematopoietic (CD45-TER119-)/non-endothelial (CD31-) cell are selected to isolate EpCAM+ cells. - In order to examine clonal proliferation capability and differentiation potential as LPCs, 5,000 to 8,000 of EpCAM+ cells are plated in 35-mm dish coated with laminin 111. Laminin 111 is diluted to 10 μg/ml in PBS. One ml of the solution is added to a 35 mm dish and incubated at room temperature for 1 h. After 6-9 days of incubation, cells are washed with PBS and fixed in 4% PFA solution at 4 °C for 10 minutes. Medium is changed at day 7 of culture. Non-specific antibody binding is blocked by Blockace and then incubated with rabbit anti-mouse CK19 and goat anti-mouse ALB for 4 h or overnight at 4 °C. Signals are visualized by Alexa488 conjugated anti-rabbit IgG and AlexaFluor555 conjugated anti-goat IgG. Nuclei are counter-stained with Hoechst33258. Cells are treated with Hoechst and secondary antibodies at the same time for 2 to 4 h at 4 °C. For staining cells in a 35 mm dish, 1 ml of PBS containing 1 μl of each antibody or Hoechst is used. After washing with PBS, images are captured on a fluorescence microscope by using 10x or 20x objective lens. For a long-term storage, a plate is mounted with Prolong Gold. Colonies containing more than 50 cells are categorized into 2 groups; bipotential colonies, which consist of ALB+ hepatocytes and CK19+ cholangiocytes, and cholangiocyte ones, which consist of only CK19+ cells. We define a cell forming bipotential colonies as LPC (Figure 3).
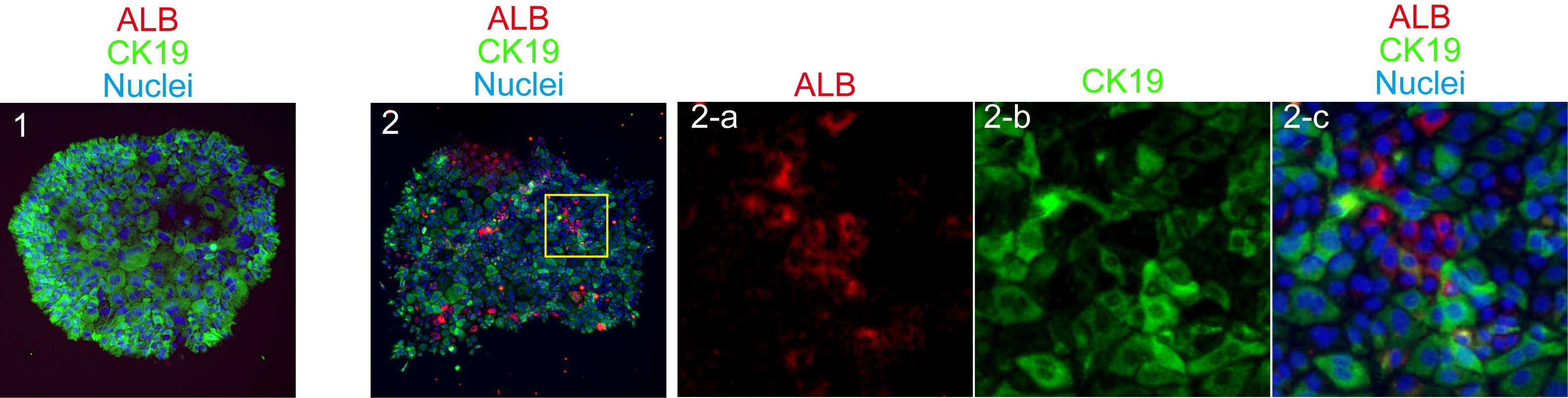
Figure 3. Typical cholangiocyte and bipotential colonies derived from a EpCAM+ cell. A colony containing only CK19+ cholangiocytes (green) is defined as “cholangiocyte colony” (panel 1), whereas a colony containing both ALB+ hepatocytes (red) and CK19+ cholangiocytes (green) is as “bipotential colony” (panel 2). These colonies are derived from EpCAM+ cells isolated from 6W mice. - For analyzing effect of microRNA (or any target molecules) on hepatocyte or cholangiocyte differentiation, microRNA mimic and small interference RNA are introduced to cells forming colonies. First, clonal culture is continued for 1 month. Each large colony is surrounded by a cloning ring and then treated with 50 μl of 0.05% trypsin at 37 °C for 10 min. Cells are resuspended in fresh culture medium and then split into 2 wells of a 96-well plate. Two days after plating, cells were transfected with negative control or microRNA mimic by using RNAiMAX. Stealth RNA can be also used to knockdown a target gene. Cells are incubated in the presence of microRNA mimic for 2 days. Cells are suspended in lysis solution provided as RLT solution in RNeasy mini kit and then total RNA was extracted to prepare first strand DNA by using a RNeasy mini kit according to the manufacturer’s instruction.
Notes
- It is expected to acquire 2 x 104 EpCAM+ cells from 6W liver.
- For vigorous stirring, use a round-shaped stirring bar rather than a typical one. If red blood cells are abundant in pellet after hyaluronidase treatment, hemolysis in 16.5 mM Tris-HCl/105 mM NH4Cl solution should be performed. If you need to increase efficiency of colony formation, add 20 μM Y27632, a ROCK inhibitor to culture medium.
Recipes
- Pre-perfusion solution
Dissolve 190 mg EGTA and 1 ml insulin (500 μg/ml) in 850 ml ddH2O containing 100 ml 10 x HBSS
Add 7 ml 1 M NaHCO3 to adjust pH7.5
Adjust the volume to 1,000 ml by ddH2O and filter it with a 0.2 μm filter - Perfusion solution
Add 1 ml insulin (500 μg/ml) to 200 ml HBSS - L-15 medium
L-15 medium is added with insulin (final concentration is 0.5 μg/ml) and gentamicin (final concentration is 50 μg/ml) before use - Hyaluronidase
350 units/μl and 25 μl is used with 100 mg Collagenase for digestion in 25 ml L-15 medium. - Culture medium
Add 10% FBS, 10-7 M Dex, 1 x ITS, 10 mM nicotinamide, 10 ng/ml EGF, 10 ng/ml HGF to DMEM/F12
Acknowledgments
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan, Grants-in-Aid for Scientific Research (C) for N. Tanimizu (25460271). This protocol was modified from Tanimizu et al. (2014).
References
- Tanimizu, N., Kobayashi, S., Ichinohe, N. and Mitaka, T. (2014). Downregulation of miR122 by grainyhead-like 2 restricts the hepatocytic differentiation potential of adult liver progenitor cells. Development 141(23): 4448-4456.
- Tanimizu, N., Nakamura, Y., Ichinohe, N., Mizuguchi, T., Hirata, K. and Mitaka, T. (2013). Hepatic biliary epithelial cells acquire epithelial integrity but lose plasticity to differentiate into hepatocytes in vitro during development. J Cell Sci 126(Pt 22): 5239-5246.
- Tanimizu, N., Nishikawa, M., Saito, H., Tsujimura, T. and Miyajima, A. (2003). Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci 116(Pt 9): 1775-1786.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Tanimizu, N. (2015). Clonal Culture of Mouse Liver Progenitor Cells. Bio-protocol 5(20): e1624. DOI: 10.21769/BioProtoc.1624.
Category
Stem Cell > Adult stem cell > Maintenance and differentiation
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link