- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Culture of Human Endometrial Epithelial Cells and Stromal Fibroblasts
Published: Vol 5, Iss 20, Oct 20, 2015 DOI: 10.21769/BioProtoc.1623 Views: 18397
Reviewed by: Andrea IntroiniBenoit ChassaingAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2243 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Purification and culture of endometrial epithelial cells (eEC) and stromal fibroblasts (eSF) from endometrial biopsies allows for downstream cell-specific in vitro studies. The utility of this protocol is the ease with which cells are purified without contamination from unwanted cell types, and the ability to use patient-paired eEC and eSF in experiments. These methods have been previously published, but here the protocol has been updated for maximum efficiency.
Keywords: Epithelial cellsMaterials and Reagents
- FalconTM 15 ml Conical Centrifuge Tubes (Thermo Fisher Scientific, catalog number: 14-959-49D )
- Cell Culture/Petri Dishes (100 x 20 mm) (Thermo Fisher Scientific, NuncTM, catalog number: 172958 )
- FalconTM 50 ml Conical Centrifuge Tubes (Thermo Fisher Scientific, catalog number: 14-432-22 )
- Costar® 24 Well Clear TC-Treated Multiple Well Plates, Bulk Pack, Sterile (Corning, catalog number: 3527 )
- Sterile Filtration Units (0.1 µm pore) (Merck Millipore Corporation, catalog number: SCVPU02RE )
- Procurement of endometrial biopsies
- Endometrial biopsies from reproductive age women (ages 28–53) were obtained as previously cited (Chen et al., 2013; Chen et al., 2014). Briefly, women who are undergoing benign gynecologic procedures are consented to donate a sample of their endometrial biopsies via the Committee on Human Research (CHR) at UCSF (CHR Protocol, catalog number: 10-02786 ). Each organization should ensure that the appropriate IRB protocols are being utilized.
- Digestion media (see Recipes)
- Collagenase I [1 gm/(249 U/mg)] (Worthington Biochemical Corporation, catalog number: LS004196 )
- Hyaluronidase from sheep testes (856 U/mg solid) (Sigma-Aldrich, catalog number: H 2251 )
Note: This product has been discontinued. A comparable substitute is Sigma-Aldrich H2126 at equal kU/ml. It is possible to use other comparable products/classes of hyaluronidase as long as the U/mg is comparable and the product has comparable bioactivity. - HBSS w/ Mg2+ and Ca2+, pH ranging 6.7-7.8 (UCSF Cell Culture Facility)
- HBSS w/o Mg2+ and Ca2+, pH ranging 6.7-7.8 (UCSF Cell Culture Facility)
- Dulbecco’s phosphate buffered saline (pH 7.2) (PBS) (UCSF Cell Culture Facility)
- Penicillin Streptomycin (Pen/Strep, 1 nM 1x working solution) (UCSF Cell Culture Facility)
- Collagenase I [1 gm/(249 U/mg)] (Worthington Biochemical Corporation, catalog number: LS004196 )
- Transfer media (see Recipes)
- Fetal bovine serum (FBS) (Charcoal/Dextran Stripped, sterile filtered, virus and mycoplasma tested) (Gemin Bio-Productsi, catalog number: 100-119 )
- MCDB-105 medium (powder) with trace elements (Sigma-Aldrich, catalog number: M6395 ) (see Recipes)
- 1N NaOH, cell culture grade (Sigma-Aldrich, catalog number: S2770 )
- ddH2O
- Pen/Strep
- Fetal bovine serum (FBS) (Charcoal/Dextran Stripped, sterile filtered, virus and mycoplasma tested) (Gemin Bio-Productsi, catalog number: 100-119 )
- Stromal cell medium (SCM) (see Recipes)
- DMEM without phenol red (Life Technologies, Gibco®, catalog number: 21063-029 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 21063-029”. - Sodium pyruvate solution (1 mM working solution) (Sigma-Aldrich, catalog number: S8636 )
- MCDB-105
- FBS
- Antibiotic Antimycotic (AB/AM, 1 nM 1x working solution) (UCSF Cell Culture Facility)
- Gentamycin (gent, 0.1 nM 1x working solution) (UCSF Cell Culture Facility)
- DMEM without phenol red (Life Technologies, Gibco®, catalog number: 21063-029 )
- Cell culture materials and reagents
- BioCoatTM Matrigel® Matrix Thin Layer 24 Well Clear Flat Bottom TC-Treated Multiwell Plate (Thin Layer 100 µg/cm2) (Corning, catalog number: 354605 )
- 40 µm sterile cell strainer (fits onto a 50 ml Falcon tube) (BD Biosciences, Falcon®, catalog number: 352340 )
Note: Currently, it is “ Corning, catalog number: 352340 ”. - Defined Keratinocyte Serum Free Media (KSFM) (Life Technologies, Gibco®, catalog number: 10785-012 ) [comes as part of a kit including the growth supplement (Life Technologies, Gibco®,catalog number: 10784-015 )]
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 21063-029 and 10784-015”. - Accutase cell detachment solution (Merck Millipore Corporation, catalog number: SCR005 )
- Red blood cell lysis buffer (see recipes)
- PBS
- 0.25% Trypsin (UCSF Cell Culture Facility)
- BioCoatTM Matrigel® Matrix Thin Layer 24 Well Clear Flat Bottom TC-Treated Multiwell Plate (Thin Layer 100 µg/cm2) (Corning, catalog number: 354605 )
Equipment
- Rotator (Multi-Rotator) (any rotator will do) (Biosan, model: Multi-bio RS-24 )
- FisherbrandTM Cell Strainers (Thermo Fisher Scientific, catalog number: 22363547 )
- Centrifuge (any appropriate centrifuge will do) (Thermo Fisher Scientific, model: Sorvall Legend RT + centrifuge)
- Incubator (Thermo Fisher Scientific, model: Forma 3110 CO2 Water Jacketed Tissue Culture Incubator)
- Serological pipettes
- Sterile Forceps, scalpels, and other surgical tools (Thermo Fisher Scientific)
Note: Stainless steel, fully autoclavable and suitable for gamma radiation sterilization. The exact catalog numbers for the set currently used are unavailable but these items are similar across vendors. - Brightfield microscope (inverted for cell culture)
Procedure
- Tissue digestion procedure
- Endometrial biopsies should be transferred to a 15 ml Falcon tube containing transfer medium in a chilled carrier (or in the presence of ice packs).
- Endometrial tissues will remain stable at 4 °C for 24 h and do not need to be processed immediately.
- Prepare a 15 ml Falcon tube with 5-7 ml 1x digestion media.
- Transfer the tissue and media into a petri dish. Use forceps and scalpels to gently pull the endometrium away from the myometrium if utilizing a full tissue section. Discard the myometrium. Endometrial biopsies procured by pipelle endometrial suction curette are easy to dissect and should not require extensive force. Cut the tissues into 1 mm3 pieces.
- Observe by brightfield microscopy. When viewed with a 50x lens, endometrial blocks will look dark with visible glandular/luminal fragments embedded within. Epithelial fragments appear to have a shiny, worm/pearl-necklace-like morphology while non-epithelial tissue pieces appear as dark shapes. The epithelial fragments will appear as if they are “embedded” in these dark shapes. Single cells are also observable at this magnification.
- Use a 2 ml-5 ml pipet to pull up the pieces and transfer to a new 15 ml Falcon tube. Centrifuge at 300 x g for 1-2 min to pellet the endometrial tissues and single cells, discard the supernatant, and resuspend in 5-10 ml 1x digestion media (10 ml for larger, two-three pipelle pass biopsies).
- Incubate on a rotator (10-20 rpm depending on the model) for 1-2 h at 37 °C (a sterile incubator can be used for this). Gently shake the tube manually every 15 min to assist with digestion.
- The digested material will now comprise mainly of single cells and epithelial fragments (which is composed of luminal epithelial sheets and glandular epithelium). Compared to pre-digestion, they will now appear free floating and not “embedded” in the dark shapes. If non-digested tissue remains, carefully pipette out these fragments by transferring the digested matter into a petri dish. Continue to A9.
- Pipette the digested matter into a 40 µm cell strainer placed on top of an open 50 ml Falcon tube.
- The flow-through contains a heterogeneous mix of leukocytes, endometrial stem cells, and stromal fibroblasts.
- Figure 1 represents a schematic summary of the digestion and separation procedure.

Figure 1. The endometrial tissue digestion and cell plating procedure
- Endometrial biopsies should be transferred to a 15 ml Falcon tube containing transfer medium in a chilled carrier (or in the presence of ice packs).
- Culturing of primary eEC
- Reverse-wash the filter into a petri dish with PBS by turning the filter upside down and using PBS to wash the retained materials off into the dish. The contents should contain luminal and glandular epithelial fragments. Incubate the petri dish with 10 ml of a 1:10 dilution of SCM in PBS. During this process, called selective attachment (Zhang et al., 1995; Kirk and Irwin, 1980; Kirk et al., 1978), eSF will attach to the plastic petri dish in the presence of serum, while epithelial fragments will not attach. Incubate for 1 h at 37 °C. Under 50x magnification, it is possible to identify contaminating tissue pieces, and remove them by gentle aspiration using a pipette.
- Collect epithelial fragments in a 15 ml Falcon conical tube using a serological pipette and spin down at 300 x g for 5 min to pellet epithelial fragments. Aspirate the 1:10 diluted SCM and wash the pellet two more times with 10 ml of defined KSFM at 300 x g for 5 min to wash out remnants of FBS.
- (Simultaneously with step B2) Add 500 µl of KSFM to a 24-well Matrigel-coated plate to rehydrate the Matrigel. Incubate at room temperature and remove the medium after 30 min. Add 500 µl of fresh KSFM to the wells.
- Reconstitute the pellet in KSFM. For the average size endometrial pipelle biopsy (one uterine pass with the pipelle), 3 ml of KSFM is appropriate for the average epithelial yield. Mix the epithelial fragments with a pipette and add 250-500 µl of medium containing epithelial fragments into each well. For the average size pipelle biopsy, there should be enough fragments to plate ~6-12 wells. When viewing the epithelial fragments, it is optimal to have 5-10 fragments per viewing field per well at 50x magnification for a 24 well plate (Figure 2A).
Note that it is important to mix the fragments after seeding each well since fragments will sediment quickly. Thus, we advise mixing of the bulk solution of fragments before pipetting into each well.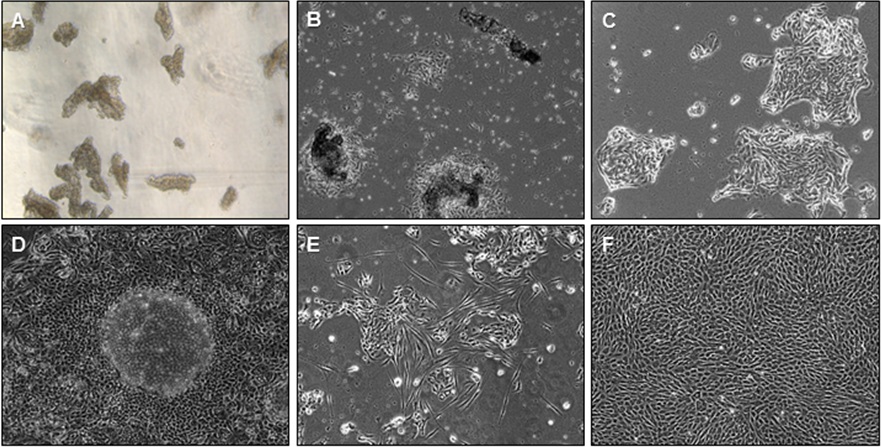
Figure 2. Bright field microscopy of endometrial cell populations. A. Epithelial sheets and fragments; B. Initial attachment of eEC onto Matrigel; C. eEC growing in island-shaped clusters; D. Confluent eEC monolayer with dome-shaped formations; E. eEC culture with eSF overgrowth; F. Confluent eSF. 50x magnification. - Glands will attach to Matrigel within 24 h (Figure 2B). Attached glands will spread out and grow into island-like clusters (Figure 2C). Eventually these cellular islands will form one major epithelial monolayer. Healthy eEC monolayer will become confluent in 5-10 days, and form dome-like structures (Figure 2D). These domes represent the eEC monolayer folding over each other.
- The appearance of spindle-like cells likely represents eSF contamination. This type of eSF contamination is common when culturing cells of epithelial origins. Studies report that 1-5% contamination is common (Pierro et al., 2001; Chen et al., 2013), but this contamination does not usually affect the eEC monolayer. eSF in the presence of a low calcium environment such KSFM medium will eventually become non-viable and detach. However, there is the possibility that eSF will propagate even in sub-optimal conditions, and overtake the eEC culture (Figure 2E). These samples are unusable and should be discarded.
- It is possible to passage the cultured cells once, but not more than that as eEC are reported to have limited expansion potential (Pierro et al., 2001; Chen et al., 2013; Chen et al., 2014). To passage eEC, remove the KSFM and incubate with Accutase (500 µl for a 24 well plate) at 37 °C for 20-30 min.
Note that trypsin-based reagents may lower eEC viability and should be avoided. After eEC is detached, wash twice in KSFM (for ease of use, it is possible to pool all the wells of cells in Accutase into a 15 ml Falcon tube). Add equivalent volumes of KSFM. Spin again at 300 x g to remove the supernatant. Resuspend the single-cell suspension in KSFM. The volume will vary based on the cell density. Plate 1 x 105 cells into each subsequent 24-well Matrigel-coated plates.
- Reverse-wash the filter into a petri dish with PBS by turning the filter upside down and using PBS to wash the retained materials off into the dish. The contents should contain luminal and glandular epithelial fragments. Incubate the petri dish with 10 ml of a 1:10 dilution of SCM in PBS. During this process, called selective attachment (Zhang et al., 1995; Kirk and Irwin, 1980; Kirk et al., 1978), eSF will attach to the plastic petri dish in the presence of serum, while epithelial fragments will not attach. Incubate for 1 h at 37 °C. Under 50x magnification, it is possible to identify contaminating tissue pieces, and remove them by gentle aspiration using a pipette.
- Culturing of Primary eSF
- Centrifuge the filtered single-cell suspension at 300 x g for 5 min (from step A9) to remove digestion media. If necessary, the pellet can be treated with red blood cell lysis buffer for 1-2 min, centrifuged and then washed twice with PBS. More than two treatments with lysis buffer are not recommended.
Note: It is not absolutely necessary to treat with lysis buffer, however blood and mucous may result in poor visualization of eSF cultures. - The pellet (containing mostly eSF, but also some leukocytes, stem cells, and endothelial cells) should then be resuspended in SCM then plated directly onto 10 cm cell culture petri dishes. The use of SCM selects for eSF proliferation while disfavoring the survival of non-eSF cells. Plate 2-3 x 105 eSF into petri dishes, or 5 x 104 cells into a 24-well plate, depending on experimental goals. eSF confluency should be achieved within 5 days (Figure 2F).
- eSF can be passaged using trypsin-based detaching reagents. Briefly, plated cells are washed once with PBS, and 0.25% trypsin is added for 5 min in the incubator to detach eSF.
- After detachment with trypsin, neutralize the trypsin with equal volumes of SCM. Centrifuge at 300 x g for 5 min and resuspend in SCM. The volume should be adjusted accordingly based on the viable cell count. eSF are now ready to be plated.
- eSF can be routinely passaged up to passages 3-4.
Note that it is possible to isolate other cell types from the digested single-cell matter through flow cytometric sorting as previously reported (Chen et al., 2014).
- Centrifuge the filtered single-cell suspension at 300 x g for 5 min (from step A9) to remove digestion media. If necessary, the pellet can be treated with red blood cell lysis buffer for 1-2 min, centrifuged and then washed twice with PBS. More than two treatments with lysis buffer are not recommended.
Troubleshooting
- My eEC fragments are not attaching to Matrigel.
- The Matrigel must be rehydrated before seeding of eEC.
- Increasing the seeding density (fragments per 50x viewing area) may help.
- Over-seeding of eEC fragments may also inhibit growth potential (adding too many fragments will prevent optimal attachment surface area, usually > 10 fragments per viewing area).
- Extend the incubation of eEC on Matrigel to 48 h before washing off non-attached cells.
- Patient use of progestin based contraceptives or the presence of chronic inflammatory endometrial disorders may affect eEC growth potential.
- The Matrigel must be rehydrated before seeding of eEC.
- My eEC culture is becoming contaminated with bacteria/yeast/fungus.
- Adding gent or AB/AM at working concentrations to KSFM can reduce exogenous contamination.
- Increasing the selective attachment incubation time or adding more SCM (1:5 dilution instead of 1:10) to the selective attachment plate since SCM contains antibiotics.
- Adding gent or AB/AM at working concentrations to KSFM can reduce exogenous contamination.
- My eEC culture has become non-viable due to significant eSF overgrowth.
- If a substantial number of free individual cells are observed immediately prior to pre-plating of eEC, perform an additional filtration step with a 40 µm cell strainer.
- Increase the selective attachment incubation time.
- If a substantial number of free individual cells are observed immediately prior to pre-plating of eEC, perform an additional filtration step with a 40 µm cell strainer.
- My eSF culture has some residual eEC in the primary culture. Is that ok?
- Some eEC fragments may not be filtered out and remain in the eSF culture. These eEC will undergo attrition in SCM media and become non-viable.
- Additional passaging of eSF to P2 before experimentation will ensure a pure eSF population.
- Some eEC fragments may not be filtered out and remain in the eSF culture. These eEC will undergo attrition in SCM media and become non-viable.
Recipes
- 2x digestion media
Combine 156 ml of HBSS w/Mg2+ and Ca2+ to the collagenase I powder
Add 3.12 ml hyaluronidase (20 kU total)
Add 3 ml of Pen/Strep (18.8 nM working concentration)
6.4 mg/ml collagenase type I
125 U/ml hyaluronidase (final 2x concentration)
Aliquot 5 ml into 15 ml Falcon tubes and freeze at -20 °C for storage up to a year
For the 1x working solution, dilute 1:1 in HBSS w/o Mg2+ and Ca2+
Note: If the tissue is large and a more concentrated digestion is desired, it is not necessary to dilute to 1x. Tissues can be put directly into 10 ml 2x digestion medium. Please also note that PBS or Transfer Media (see below) can substitute for HBSS w/o Mg2+ and Ca2+ as the diluent. - MCDB-105 medium with trace elements
Combine a bottle of MCDB-105 in 1 L of ddH2O
Add 15 ml of 1 N NaOH, which should turn the solution pink
Sterile filter the solution - Transfer media
90% MCDB-105
10% FBS
1x Pen/Strep - Stromal cell medium (SCM)
Add 5 ml of sodium pyruvate to 500 ml DMEM
67.5% DMEM
22.5% MCDB-105
10% FBS
1x Antibiotic/Antimycotic (1 nM working concentration) and 1x gent (0.1 nM working concentration - Red blood cell lysis buffer
Prepared using ddH2O
0.155 M NH4Cl
0.01 M KHCO3
0.1 mM EDTA (pH 7.3)
Acknowledgments
We would like to acknowledge Dr. Linda C. Giudice, M.D., Ph.D., Dr. Juan C. Irwin, MD, Ph.D., and Kim Chi Vo. This work was funded in part by the F32HD074423 (JCC), the K99AI104262 (NRR), and a grant from the NIH, University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research, P30-AI027763 (NRR).
References
- Chen, J. C., Erikson, D. W., Piltonen, T. T., Meyer, M. R., Barragan, F., McIntire, R. H., Tamaresis, J. S., Vo, K. C., Giudice, L. C. and Irwin, J. C. (2013). Coculturing human endometrial epithelial cells and stromal fibroblasts alters cell-specific gene expression and cytokine production. Fertil Steril 100(4): 1132-1143.
- Chen, J. C., Johnson, B. A., Erikson, D. W., Piltonen, T. T., Barragan, F., Chu, S., Kohgadai, N., Irwin, J. C., Greene, W. C., Giudice, L. C. and Roan, N. R. (2014). Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts. Hum Reprod 29(6): 1255-1270.
- Kirk, D. and Irwin, J. C. (1980). Normal human endometrium in cell culture. Methods Cell Biol 21B: 51-77.
- Kirk, D., King, R. J., Heyes, J., Peachey, L., Hirsch, P. J. and Taylor, R. W. (1978). Normal human endometrium in cell culture. I. Separation and characterization of epithelial and stromal components in vitro. In Vitro 14(8): 651-662.
- Pierro, E., Minici, F., Alesiani, O., Miceli, F., Proto, C., Screpanti, I., Mancuso, S. and Lanzone, A. (2001). Stromal-epithelial interactions modulate estrogen responsiveness in normal human endometrium. Biol Reprod 64(3): 831-838.
- Spitzer, T. L., Rojas, A., Zelenko, Z., Aghajanova, L., Erikson, D. W., Barragan, F., Meyer, M., Tamaresis, J. S., Hamilton, A. E., Irwin, J. C. and Giudice, L. C. (2012). Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod 86(2): 58.
- Zhang, L., Rees, M. C. and Bicknell, R. (1995). The isolation and long-term culture of normal human endometrial epithelium and stroma. Expression of mRNAs for angiogenic polypeptides basally and on oestrogen and progesterone challenges. J Cell Sci 108 ( Pt 1): 323-331.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chen, J. C. and Roan, N. (2015). Isolation and Culture of Human Endometrial Epithelial Cells and Stromal Fibroblasts. Bio-protocol 5(20): e1623. DOI: 10.21769/BioProtoc.1623.
Category
Immunology > Mucosal immunology > Genitourinary tract
Cell Biology > Cell isolation and culture > Cell isolation
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










