- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Intravenous Tomato Lectin Injection to Assess Functional Vasculature
Published: Vol 5, Iss 20, Oct 20, 2015 DOI: 10.21769/BioProtoc.1619 Views: 12903
Reviewed by: Ningfei AnAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Systematic Analysis of Smooth Muscle and Cartilage Ring Formation during Mouse Tracheal Tubulogenesis
Haoyu Wu [...] Wenguang Yin
Jul 5, 2023 1537 Views

Protocol for the Implantation of Scaffolds in a Humanized Mouse Cutaneous Excisional Wound Healing Model
Dina Gadalla [...] David G. Lott
Sep 20, 2024 1457 Views

Dissection and Whole-Mount Immunofluorescent Staining of Mouse Hind Paw Muscles for Neuromuscular Junction Analysis
Rebecca L. Simkin [...] James N. Sleigh
May 20, 2025 3943 Views
Abstract
Pluripotent stem cells have recently allowed for the development of tissue models for the various organ systems within the body. These models allow scientists to study organ function, physiology, embryology, and even pathologic processes. Studies on tissue can be done in vitro and/or transplanted into animal models for studies in vivo. Recently, our lab developed a model of human small intestine derived from human pluripotent stem cells which when transplanted in vivo, matured into an intestinal structure similar to that of adult intestine. The maturity of the transplanted human intestinal tissue was dependent upon the development of an adequate blood supply primarily from the murine host. In order to better study the developed vascular network within our transplanted intestinal tissue, we injected Fluorescein labeled Lycopersicon esculentum (tomato) lectin into the mouse tail vein (Watson et al., 2014). Using the property of this lectin to bind to the endothelium, we were able to visualize the vasculature within the transplant.
Materials and Reagents
- 1 ml syringes
- 30 gauge needles
- Small surgical kit (scissors, ringed forceps, needle driver/holder)
- Immune deficient NOD-SCID IL-2Rγnull (NSG) mice, 8-16 weeks of age (bred in house), transplanted with a bioengineered intestine (maturation of transplant then allowed for 6-8 weeks)
- Fluorescein labeled Lycopersicon esculentum lectin (Tomato-Lectin) (Vector Laboratories, catalog number: FL-1171 )
- Phosphate-buffered saline (PBS)
- Isopropyl Alcohol
- 4% Paraformaldehyde in PBS (PFA)
- Water-based optical clearing solution (Ke et al., 2014)
Equipment
- Dissecting Microscope (Leica Microsystems)
Procedure
- Dissolve Tomato-Lectin in PBS at a final concentration of 2 mg/ml.
- Clean the proximal portion of a transplanted NSG mouse tail with isopropyl alcohol.
- Draw up 200 μl of the Tomato-lectin solution into a 1 ml syringe equipped with a 30 gauge injection needle.
- Using proper restraint, hold the tail of the mouse so that the lateral tail vein is visible and then inject the vein with 200 μl of Tomato-lectin solution. Place the mouse into a clean, warm area for recovery. The method for proper tail vein injection is described elsewhere (Machholz et al., 2012).
- 15 to 30 min after injection, sacrifice the mice using CO2 exposure and confirm the euthanasia by cervical dislocation. All animal euthanasia must be performed in accordance with the IACUC guidelines and an approved animal protocol within the institution.
- Collect the appropriate tissues for histology at this time (transplanted tissue plus intestines for comparison) and place the tissues in ice-cold PBS. Fix collected tissue in 4% PFA at 4 °C overnight.
- Rinse fixed tissues five times in PBS and then process in optical clearing solution according to the following protocol (Ke et al., 2013).
- Image cleared tissues under a confocal microscope to visualize the Tomato-lectin (Excitation 494 nm; Emission 521 nm) which reflects the functional vasculature (Figure 1).
Representative data
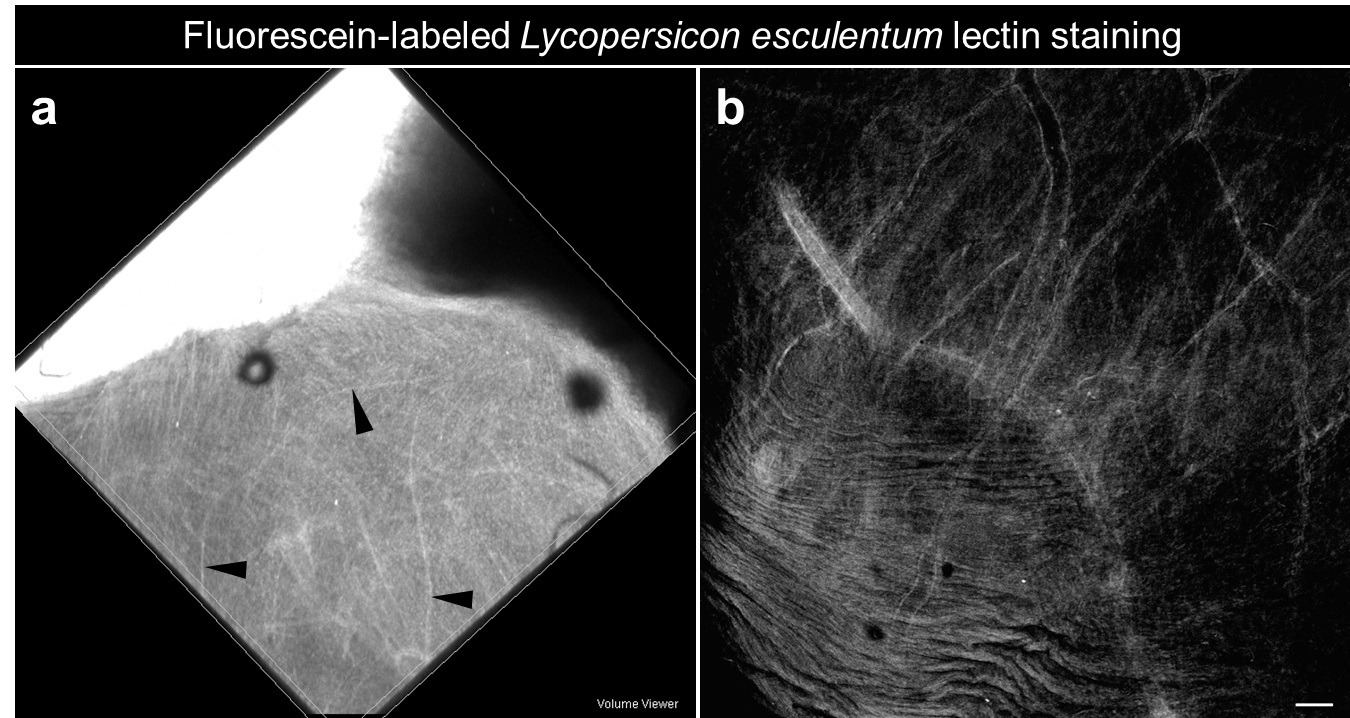
Figure 1. Functional vasculature within a human small intestine derived from human pluripotent stem cells and transplanted in vivo under the kidney capsule of an immunocompromised mouse. a. Whole mount of bioengineered intestine within the kidney following fluorescein-labeled Lycopersicon esculentum lectin perfusion (Black arrowheads: blood vessels). b. Confocal imaging on whole mount reveals functional fluorescent vasculature within the engraftment (scale bars 50 μm).
Notes
- Freshly collected tissues can be directly imaged under a fluorescent stereoscope equipped with a mercury lamp and a FITC filter.
- Fixed tissue can be kept several days in clearing agent until imaging at room-temperature.
- This protocol allows for imaging of the functional vasculature within transplanted tissues and/or intestinal tissues.
- This protocol was adapted from work previously performed with “human liver buds” transplanted into a murine host and using 1% tetramethylrhodamine-, FITC- and Texas-Red-conjugated dextran via tail vein injection (Takebe et al., 2014).Intravital imaging is possible using a variety of fluorescent-labeled products which can be combined with immunofluorescence staining.
Acknowledgments
This project was supported in part by US National Institutes of Health (NIH) grants NIH-R01DK083325 (M.A.H), NIH P30 DK078392 (Digestive Health Center, Cincinnati Children’s Hospital Medical Center), and NIH UL1RR026314 (Clinical and Translational Science Awards (CTSA), University of Cincinnati).
References
- Ke, M. T., Fujimoto, S. and Imai, T. (2013). SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci 16(8): 1154-1161.
- Ke, M., Fujimoto, S. and Imai, T. (2014). Optical clearing using seeDB. Bio-protocol 4(3): e1042.
- Machholz, E., Mulder, G., Ruiz, C., Corning, B. F. and Pritchett-Corning, K. R. (2012). Manual restraint and common compound administration routes in mice and rats. J Vis Exp(67).
- Takebe, T., Zhang, R. R., Koike, H., Kimura, M., Yoshizawa, E., Enomura, M., Koike, N., Sekine, K. and Taniguchi, H. (2014). Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc 9(2): 396-409.
- Watson, C. L., Mahe, M. M., Munera, J., Howell, J. C., Sundaram, N., Poling, H. M., Schweitzer, J. I., Vallance, J. E., Mayhew, C. N., Sun, Y., Grabowski, G., Finkbeiner, S. R., Spence, J. R., Shroyer, N. F., Wells, J. M. and Helmrath, M. A. (2014). An in vivo model of human small intestine using pluripotent stem cells. Nat Med 20(11): 1310-1314.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Watson, C. L., Mahe, M. M. and Helmrath, M. A. (2015). Intravenous Tomato Lectin Injection to Assess Functional Vasculature. Bio-protocol 5(20): e1619. DOI: 10.21769/BioProtoc.1619.
Category
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









