- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Chick Neural Tube Explant Culture
Published: Vol 5, Iss 19, Oct 5, 2015 DOI: 10.21769/BioProtoc.1608 Views: 11053
Reviewed by: Oneil G. BhalalaPamela MaherGeoff Lau

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vivo Chick Chorioallantoic Membrane (CAM) Angiogenesis Assays
Zhenguo Chen [...] Xiaochun Bai
Sep 20, 2013 38746 Views

Standardized Culture of Skin Fibroblasts From Punch Biopsies for Germline DNA Isolation in Myeloid Malignancies: A Practical Bedside-to-Laboratory Approach
Parampreet Kour [...] Pulkit Rastogi
Oct 5, 2025 1418 Views

Electroporation of Whole-Mount Postnatal Rodent Retinas for Advanced Functional Assays
Chien-Ting Huang [...] Chih-Tien Wang
Jan 20, 2026 154 Views
Abstract
The neural tube explant culture technique allows in vitro culturing of small pieces of neural tissue isolated from e.g., chick or mouse embryonic tissue in a matrix of collagen for defined periods of time. This method can be used to study the effects of defined molecules on developmental processes such as neural progenitor proliferation and neuronal differentiation and/or survival. Since the explant material can also be prepared from embryonic tissue electroporated with expression vectors, this technique can be adapted to study gene function in the presence of specific environmental signals. Different regions of the neural tube can also be isolated during the dissection step, allowing specific regions of the neural tube to be studied separately. Here, we present a neural tube explant culture method that we have used in several studies (Dias et al., 2014; Lek et al., 2010; Vallstedt et al., 2005).
Materials and Reagents
- 10 cm Petri dish (SARSTEDT AG & Co, catalog number: 82.1473 )
- Glass Pasteur pipette 150 mm (VWR International, catalog number: 612-1701 )
- Transfer pipette (SARSTEDT AG & Co, catalog number: 86.1174 )
- Tungsten wire (Goodfellow, catalog number: w005160 )
- 10 ml syringe (HARTMANN USA, Omnifix®, catalog number: 4617100V )
- Needle 20 G (Henke Sass Wolf, catalog number: 4710009040 )
- Fresh fertilized chick eggs (ideally from a local supplier)
- L-15 medium (Life Technologies, catalog number: 11415-049 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 11415-049”. - Dispase I (Roche Diagnostics, catalog number: 04942086001 )
- Heat inactivated Fetal bovine serum (FBS) ( Life Technologies, GibcoTM, catalog number: 10106-169 )
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 10106-169 ”. - Dulbecco’s modified eagle’s medium 10x (MEM) (Sigma-Aldrich, catalog number: D2429 )
- Sodium hydroxide (NaOH) (EMD Millipore Corporation, catalog number: 1.06469.1000 )
- Collagen (3.1 mg/ml) (Pure Col) (Advanced BioMatrix, catalog number: 5005-B )
- Sodium bicarbonate 7.5% (BIC) (Life Technologies, catalog number: 25080 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 25080”. - DMEM/F-12, GlutaMAXTM supplement (Life Technologies, catalog number: 31331-028 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 31331-028”. - Neurobasal medium (Life Technologies, catalog number: 21103-049 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 21103-049”. - Albumin from bovine serum (BSA) (Sigma-Aldrich, catalog number: A7906 )
- Glutamax (Life Technologies, catalog number: 35050-038 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 35050-038”. - Penicillin Streptomycin (PEST) (Life Technologies, catalog number: 15140-122 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 15140-122”. - N2-supplement (Life Technologies, catalog number: 17502-048 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 17502-048”. - B27-supplement (Life Technologies, catalog number: 17504-044 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 17504-044”. - 2-mercaptoethanol (Sigma-Aldrich, catalog number: M3148 )
- Dispase I solution (see Recipes)
- Bedding solution (see Recipes)
- Growing media (see Recipes)
Equipment
- Humidified, 38 °C egg incubator (Grumbach, catalog number: 8012 )
- Humidified, 37 °C tissue culture incubator (Thermo Fisher Scientific, catalog number: 51030287 )
- 16 °C incubator (Gemini BV Laboratory,Liebherr, catalog number: 4294 F1-1 )
- Dissection microscope and light source (ZEISS, models: Zeiss Stemi SV11 and Zeiss KL1500LCD )
- Borosilicate glass capillaries (Harvard Apparatus, catalog number: GC100TF-10 )
- Nunc 4-well dishes for IVF (Thermo Fisher Scientific, catalog number: 144444 )
- Flaming/brown micropipette puller (SUTTER, catalog number: P-97 )
- Needle holder (Fine science tools, catalog number: 26016-12 )
- Tweezers (Fine science tools, catalog number: 11252-20 )
- Micro-scissors (Fine science tools, catalog number: 15000-00 )
- Dissection scissors (Fine science tools, catalog number: 14088-10 )
- Mouth aspirator system/Aspirator tube assemblies for calibrated microcapillary pipettes (Sigma-Aldrich, catalog number: A5177-5EA )
Procedure
- Isolation of chick embryos
- Store fertilized eggs at 16 °C (up to a week) until start of the experiment.
- Place eggs horizontally and incubate in a humidified chamber at 38 °C until they reach the desired Hamburger-Hamilton (HH) embryonic stage (Hamburger and Hamilton, 1992). We have used this protocol successfully with chick embryos from embryonic stages HH10-25.
- Make a hole in the pole region of the egg and remove approximately 5 ml of albumin (egg white) using a 10 ml syringe-needle to lower down the embryo inside the egg.
- Cut an oval window on the top of the egg using dissection scissors.
- Remove the embryo from the egg. Use a pair of tweezers to hold the membrane near the embryo and dissection scissors to cut the membrane around the embryo (see Video 1 for steps A3-5). Transfer the embryo with attached membranes into a 10 cm Petri dish on ice containing cold L-15 medium. Remove embryos from all incubated eggs before proceeding to embryo dissection.
- Place the Petri dish containing the embryos under a dissection microscope, remove the membranes surrounding the embryos using tweezers and a dissection micro-scissor (Figure 1a-b) and transfer them to new cold L-15 medium on ice with a transfer pipette.
- Somite-stage the embryos and keep embryos of the desired developmental stage (Hamburger and Hamilton, 1992).
- Store fertilized eggs at 16 °C (up to a week) until start of the experiment.
- “Raw” embryo dissection
Note: From this point onwards, all dissection steps of the embryonic tissue will be performed at RT under dissection microscope.- Place an embryo in a Petri dish containing ice-cold L-15 medium. With a straight tungsten needle keep the embryo immobilized and with a hook-shaped tungsten needle remove as much mesenchyme as possible without damaging the neural tube (see Figure 1b-d).
- Cut the region of the neural tube of interest (e.g., Figure 1e) using the hook-shaped needle and transfer the neural tube piece to a four-well plate on ice containing L15 medium using a glass Pasteur pipette.
- Repeat steps B1-2 to remaining embryos, one at the time. If suitable, can be pooled to the same well on the four-well plate.
- Place an embryo in a Petri dish containing ice-cold L-15 medium. With a straight tungsten needle keep the embryo immobilized and with a hook-shaped tungsten needle remove as much mesenchyme as possible without damaging the neural tube (see Figure 1b-d).
- “Fine” embryo dissection
- Pull borosilicate glass capillaries using a micropipette puller and cut the tip of the needle with tweezers. Typically, a tip of approximately 0.5 mm in diameter allows an easy manipulation of the dissected tissue (explant).
- Using a four-well plate prepare a Dispase I solution well, a 10% FBS/L-15 solution well, and two L-15 medium wells (washing wells). Use a volume of solution sufficient to cover the dissected tissue (500 µl of solution per well is usually sufficient). Keep at room-temperature (RT). Transfer the dissected embryos from step B with a glass Pasteur pipette sequentially through the four wells with an incubation period of 5 min/well at RT. Keep the tissue in the last washing well and place it on ice. Do not incubate the tissue in the Dispase I solution for more than 5 min.
- Coat two 10 cm Petri dishes with ~2 ml of FBS and incubate for 2 min at RT. Remove FBS and add ice-cold L-15 medium. Keep dishes on ice.
- Transfer one tissue piece to the coated Petri dish and remove the remaining mesenchymal tissue attached to the neural tube using the tungsten needles. Use a straight-shaped needle to hold the tissue and the hook-shaped needle to remove the remaining mesenchyme. Once all mesenchyme is removed the tissue should look “shiny” and smooth (see Figure 1f). Use the hook-shaped tungsten needle to cut the desired region of the neural tube to be cultured (e.g., ventral midline, Figure 1g, intermediate or dorsal region of the neural tube).
- Transfer the dissected tissue (explant) with a glass capillary (prepared in step C1) in a mouth aspirator tube to a four-well plate coated with FBS and containing L-15 medium (~600 µl) on ice. Change to a new coated Petri dish after every second tissue dissection to make sure the media in the Petri dish is always cold.
- Pull borosilicate glass capillaries using a micropipette puller and cut the tip of the needle with tweezers. Typically, a tip of approximately 0.5 mm in diameter allows an easy manipulation of the dissected tissue (explant).
- Tissue incubation
- Prepare the bedding solution according to Recipes.
- Add a 16 µl drop to the bottom of the wells of a four-well plate. Incubate in a humidified tissue culture incubator at 37 °C for 25-30 min, until the bubble becomes slightly “milky” white (Figure 1h, top left well).
- Add 2 drops of ~50 µl of bedding solution to the lid of a 10 cm Petri dish. Wash the explants by transferring them with a glass capillary in a mouth aspirator tube from one drop of bedding to the other.
- Add ~20 µl of bedding solution on top of the dried bedding drop from step D2 and transfer the washed explants from step D3 to the new drop of bedding. The explant will sink to the top of the dried drop. Position the explant using the tungsten needles (see Figure 1i).
- Incubate ~30-60 min in a humidified tissue culture incubator at 37 °C until the bedding drop hardens. Add 650 µl/well of growing medium and incubate at 37 °C. Soluble factors can be added at any time to the growing medium according to experimental setup. Culture the explants for the desired period of time according to the experimental setup.
- Prepare the bedding solution according to Recipes.
Representative data
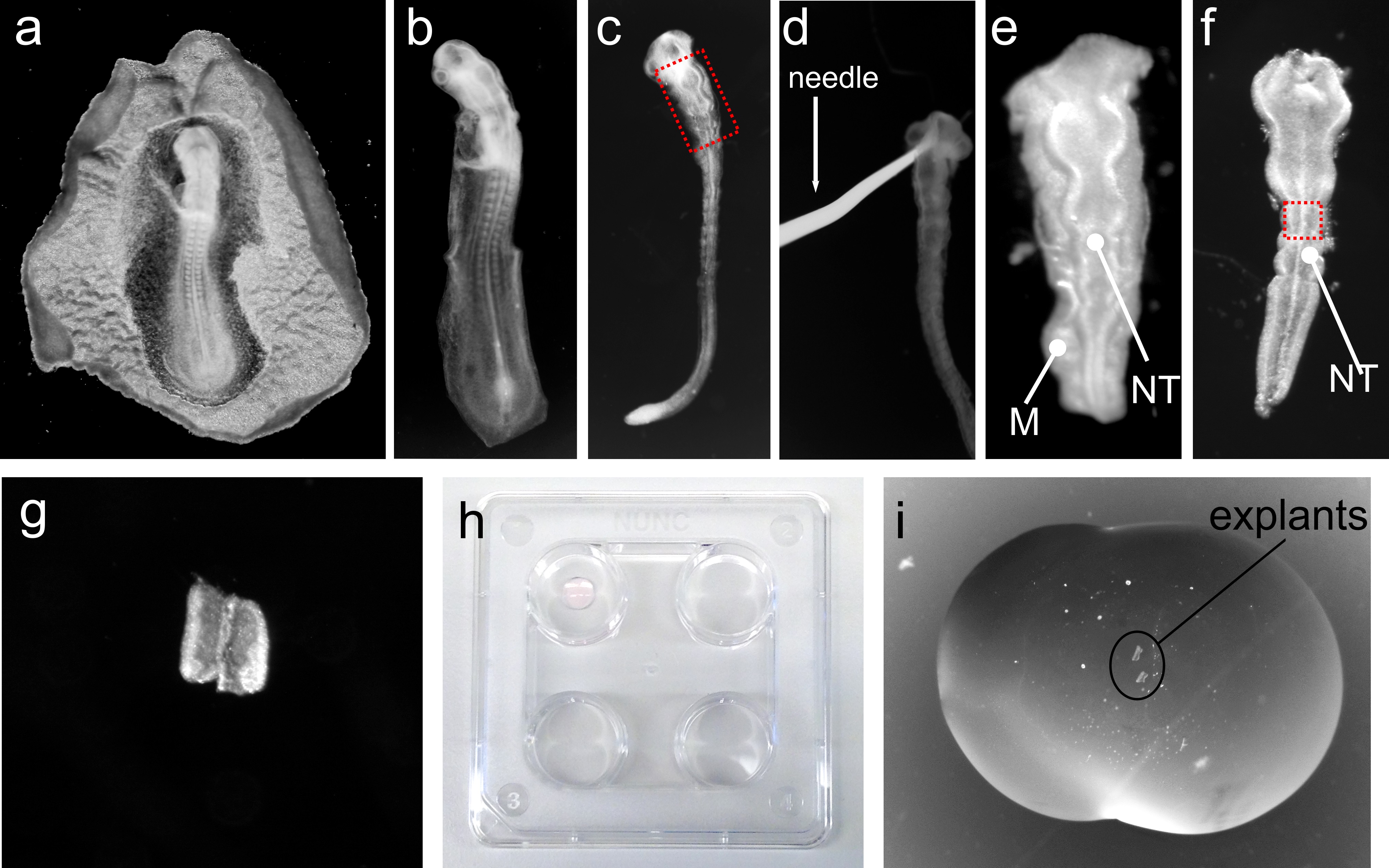
Figure 1. Representative images of different steps of chick neural tube explant technique. a. Chick embryo isolated from egg; b. Chick embryo isolated from surrounding membranes; c. Chick embryo after “raw” dissection step; d. Tungsten needle holding “raw” dissected embryo; e. Dissected hindbrain region of the neural tube of embryo shown in c; Boxed region in c corresponds to the region shown in e; f. Neural tube after fine dissection of neural tube shown in e. Note that the mesenchyme (M) surrounding the neural tube (NT) in e is now completely removed; g. Isolated ventral region neural tube, corresponding approximately to the boxed region in f; h. 4-well plate containing a bubble of collagen on top left well; i. Bubble of collagen containing 2 isolated explants of the neural tube.
Recipes
- Dispase I solution
Prepare a stock solution by dissolving Dispase I in water to a final concentration of 5 mg/ml
Aliquot the solution and store at -20 °C up to one year
Prepare a working solution of Dispase I by diluting the stock solution in L-15 medium to a final concentration of 1 mg/ml - Bedding solution
Note: This solution should be freshly prepared.
Add 8.64 µl of 2.5 M NaOH to 200 µl of MEM 10x and vortex
The solution will turn into a pink color
Add 1.8 ml of collagen (3.1 mg/ml) and mix
The solution will turn to an apricot color
Add 88 µl of BIC and mix
The final solution should have a pale pink color
Stored the solution on ice - Growing media
Mix together 250 ml of DMEM/F12 + Glutamax medium with 250 ml of Neurobasal medium, 2.5 ml of Glutamax, 2.5 ml of N2-supplement, 5 ml of B27-supplemet, 1.3 ml of a BSA stock solution at 10 mg/ml, 5 ml of PEST and 4 µl of 2-mercaptoethanol. Stored at 4 °C up to one month
Acknowledgments
Funding for this work was provided by Cancerfonden and Karolinska Institutet. This protocol was adapted from procedures published in Vallstedt et al. (2005) and Dias et al. (2014).
References
- Dias, J. M., Alekseenko, Z., Applequist, J. M. and Ericson, J. (2014). Tgfbeta signaling regulates temporal neurogenesis and potency of neural stem cells in the CNS. Neuron 84(5): 927-939.
- Hamburger, V. and Hamilton, H. L. (1992). A series of normal stages in the development of the chick embryo. 1951. Dev Dyn 195(4): 231-272.
- Lek, M., Dias, J. M., Marklund, U., Uhde, C. W., Kurdija, S., Lei, Q., Sussel, L., Rubenstein, J. L., Matise, M. P., Arnold, H. H., Jessell, T. M. and Ericson, J. (2010). A homeodomain feedback circuit underlies step-function interpretation of a Shh morphogen gradient during ventral neural patterning. Development 137(23): 4051-4060.
- Vallstedt, A., Klos, J. M. and Ericson, J. (2005). Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron 45(1): 55-67.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Alekseenko, Z., Andersson, E. and Dias, J. M. (2015). Chick Neural Tube Explant Culture. Bio-protocol 5(19): e1608. DOI: 10.21769/BioProtoc.1608.
Category
Neuroscience > Development > Explant culture
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










