- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Rhizosphere Bacterial Communities from Soil
Published: Vol 5, Iss 16, Aug 20, 2015 DOI: 10.21769/BioProtoc.1569 Views: 27210
Reviewed by: Zhaohui LiuYurong XieAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bacterial Pathogen-mediated Suppression of Host Trafficking to Lysosomes: Fluorescence Microscopy-based DQ-Red BSA Analysis
Mădălina Mocăniță [...] Vanessa M. D'Costa
Mar 5, 2024 2902 Views

Purification of Native Dentilisin Complex from Treponema denticola by Preparative Continuous Polyacrylamide Gel Electrophoresis and Functional Analysis by Gelatin Zymography
Pachiyappan Kamarajan [...] Yvonne L. Kapila
Apr 5, 2024 2095 Views

In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
Arijit Mukherjee [...] Sanjay Swarup
Nov 5, 2025 1671 Views
Abstract
Rhizosphere bacterial communities have become a major focal point of research in recent years, especially regarding how they affect plants and vice versa (Philippot et al., 2013). Changes in microbial density and diversity within the rhizosphere occur in a spatial temporal manner. The soil zone closest to the plant roots has the most density and diversity of microbes (Clark, 1940). The lack of methods to consistently isolate rhizosphere samples in a spatially defined manner is a major bottleneck in rhizosphere microbiology. We hypothesized that microbes with increasing affinities to and distance from the plant root can be isolated using increasing strengths of physical disruption. Sonication is an excellent choice due to the ability to gently remove rhizosphere soil and bacterial biofilms without damaging plant roots (Doi T et al., 2007; Bulgarelli et al., 2012; Lundberg et al., 2012). In addition, simply increasing the time of sonication can increase the amount of physical force. We used such an approach to consistently isolate microbial communities with different affinities to the soybean roots (White et al., 2014). This article describes the use of successive sonication to isolate distal, middle, and proximal soil from the rhizosphere of soybean roots.
Materials and Reagents
- Soybean seedlings (Glycine max) in the vegetative stage (~ V3 to V5 period)
- Soil with a history of soybean cultivation
- dH2O
- K2HPO4 (VWR International, catalog number: BDH0266-500 g )
- KH2PO4 (VWR International, catalog number: BDH0268-500 g )
- NaCl (Sigma-Aldrich, catalog number: S7653-1 kg )
- Tween-20 (Sigma-Aldrich, catalog number: P9416-100 ml )
- Phosphate buffered saline Tween 20 (PBST) (see Recipes)
Equipment
- Razor blade
- Tweezers
- 15 ml conical-bottom polypropylene centrifuge tubes (3 per sample) (VWR International, catalog number: 89039-670 )
- 50 ml conical-bottom polypropylene centrifuge tubes (3 per sample) (VWR International, catalog number: 21008-940 )
Note: Needed if plant roots are too large for 15 ml centrifuge tubes. - Styrofoam raft to suspend centrifuge tubes in sonicator (homemade)
- Sonicator (Input: 117 V-50-60HZ 1ϕ, Output: 70 W 42KHZ +/-6%) (Thermo Fisher Scientific, model: FS20 )
- Centrifuge with a fixed angle rotor for 15 and 50 ml conical bottom tubes at 4 °C capable of at least 5,000 x g relative centrifugal force (120 V 12 A 60 Hz 1,300 W) (Example: Eppendorf, model: 5804R 15 amp version)
Procedure
- Either directly sow plant seeds or plant seedlings into soil of interest and allow seeds/seedlings to grow for desired amount of time (minimum of 1 week suggested for soybean plants).
Notes:- Although larger roots (ex. mature tree roots) are not recommended for this procedure, representative samples of the root system can be used depending on the research question.
- Amount of growth time depends on the research focus, for example the impact of a particular root exudate or the plant growth stage on the soil microbial community.
- Although larger roots (ex. mature tree roots) are not recommended for this procedure, representative samples of the root system can be used depending on the research question.
- Carefully remove plant seedlings by saturating the soil with dH2O or gently loosening the soil by hand to avoid damage to the roots.
Note: Using an excessive amount of dH2O during saturation (i.e. resulting in a soil consistency thinner than mud) risks a loss of sample size and rhizosphere bacteria. - Submerge the roots in a still pool of dH2O and gently shake the roots (as if painting a picture or dunking a teabag) to remove the larger soil particles. Skip this step if plant seedlings were removed by soil saturation in the previous step. See Figure 1 for example of soybean roots before and after the removal of large soil particles.

Figure 1. Thirty-six day old soybean roots before (A) and after (B) submersion in a still pool of dH2O to remove large soil particles. The amount of soil clinging to the plant roots can vary depending on soil properties, the root architecture, and the size(s) of the plant roots. - Use a razor blade to sever the plant roots (cutting near the plant stem).
- Place the severed roots into separate, labeled 15 ml centrifuge tubes filled with 10 ml of PBST, ensuring they are completely submerged (may use tweezers to gently push roots deeper into the tube).
Notes:- Roots should be placed into the centrifuge tube vertically.
- Ensure the centrifuge tube is not packed with the root sample. The number of roots placed into one tube depends on root size and/or the desire to keep root samples separate (ex. pooling all roots from one plant together, pooling multiple roots from several plants together, or keeping each root from one plant separate). Overly large roots, or too many roots in one tube, will lead to poor sample isolation whereas tiny roots, or too few roots in one tube, will yield a miniscule sample size.
- For seedlings with larger root systems, use a 50 ml centrifuge tube filled with 45 ml of PBST in this step and all subsequent steps. See Figure 2 for demonstrative sample of an acceptable amount of roots in a single tube.

Figure 2. Soybean roots submerged in 10 ml of PBST within a 15 ml centrifuge tube - Roots should be placed into the centrifuge tube vertically.
- Firmly secure the centrifuge tube lids, then place the tubes in a floating raft within a sonicator filled with dH2O.
Note: Ensure the centrifuge tubes do not touch the bottom or sides of the sonicator (see Figure 3 for demonstrative diagram).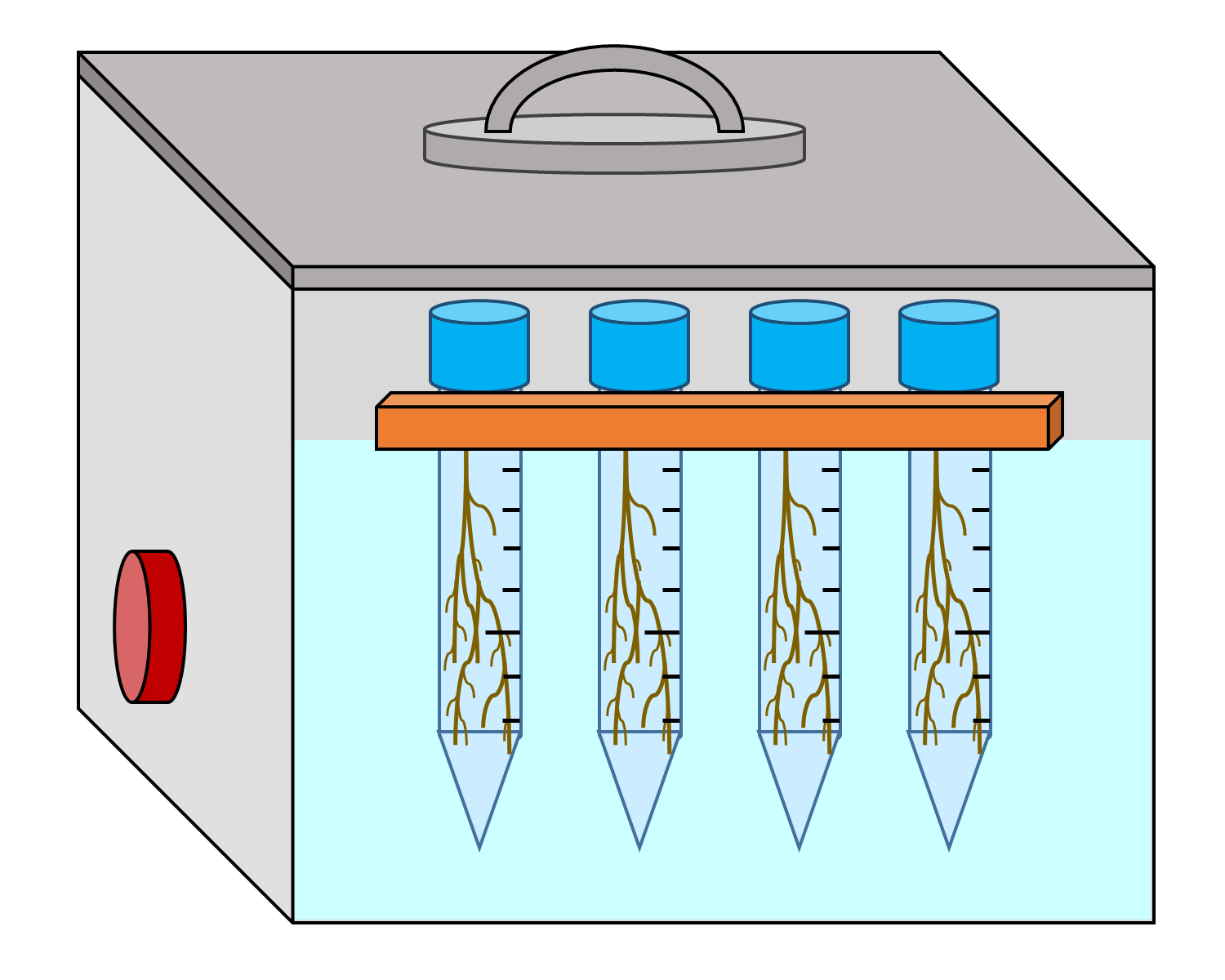
Figure 3. Diagram demonstrating how to properly load samples and floating raft into the sonicator filled with dH2O. Centrifuge tubes should be submerged up to the 10 or 45 ml line (dependent on if a 15 or 50 ml centrifuge tube was used). Tubes should not touch the bottom or edges of the sonicator. - Subject the centrifuge tubes to sonication for 60 sec, then turn off the sonicator (see Figure 4 for sonication summary).
Notes:- This sonication yields the rhizosphere soil furthest from the plant root or soil with least affinity to the plant root, noted as the “distal soil” sample.
- Use the same sonication time for both the 15 and 50 ml centrifuge tubes.
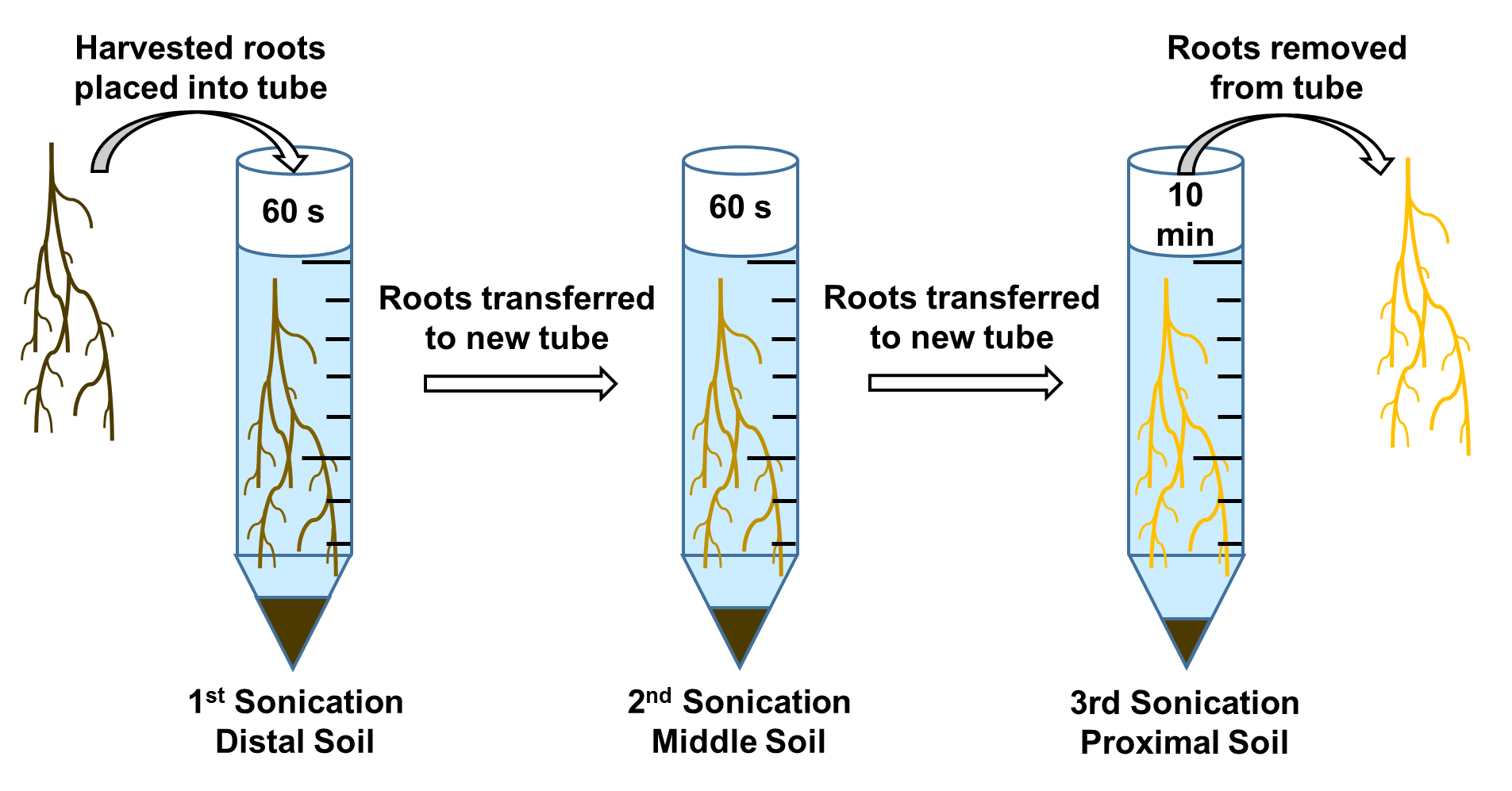
Figure 4. Diagram of successive sonication procedure for isolation of distal, middle, and proximal soil samples from plant roots. Distal soil samples consist of the rhizosphere soil furthest from and with least affinity to the plant root. Middle soil samples consist of the rhizosphere soil that is closer to and with relatively less affinity the plant root. Proximal soil samples consist of the rhizosphere soil closest to and with highest affinity the plant root. Image adapted from a previous article (White et al., 2014). - This sonication yields the rhizosphere soil furthest from the plant root or soil with least affinity to the plant root, noted as the “distal soil” sample.
- Using tweezers, gently remove the root(s) from the current centrifuge tube(s) and transfer into a new, labeled centrifuge tube (or tubes) containing 10 ml of fresh PBST.
Note: Keep roots/samples separated in the same manner used for the first sonication. Do not pool roots/samples from different centrifuge tubes together. - Firmly secure the centrifuge tube lids, place the tubes in the floating raft within the sonicator, and subject the tubes to sonication for 60 sec. Then turn off the sonicator.
Note: This sonication yields the rhizosphere soil that is closer to the plant root, noted as the “middle soil” sample. - Using tweezers, gently remove the root(s) from the current centrifuge tube(s) and transfer into a new, labeled 15 ml centrifuge tube (or tubes) containing 10 ml of fresh PBST.
Note: Again, keep roots/samples separated in the same manner used for the first sonication. Do not pool roots/samples from different centrifuge tubes together. - Firmly secure the centrifuge tube lids, place the tubes in the floating raft within the sonicator, and subject the tubes to sonication for 10 min. Then turn off the sonicator.
Note: This sonication yields the rhizosphere soil closest to the plant root including any biofilms, noted as the “proximal soil” sample. At this point, soil should not be visible on the plant root. - Using tweezers, gently remove the root(s) from the current centrifuge tube(s) and either discard the roots or place them into a new, labeled centrifuge tube (or tubes) filled with fresh PBST, then store the tubes at 4 °C until needed. Harvested samples may then be immediately used for bacterial cultivation or further processed for DNA or RNA isolation. If seeking to isolate DNA or RNA, complete the next 2 steps of the protocol. For bacterial cultivation, promptly subject the samples to a series of 6 to 10 fold dilutions using sterile dH2O and select several of these dilutions for plating (dilutions >10-3 recommended). When plating the chosen dilutions, ensure the appropriate nutrient medium (or media) is chosen. One hundred microliters of the chosen dilution should be dispensed onto the center of the petri dish and spread across the media using a flame-sterilized glass spreader. The petri dish should then be inverted and incubated under the ideal cultivating conditions (i.e. time and temperature). See Figure 5 for an example of bacterial cultivation via petri dish.
Notes:- Distal, middle, and proximal soil samples are all useful for bacterial cultivation. However, proximal soil samples are preferable as they contain the bacteria that most likely affect the plant directly and vice versa.
- Possible media for bacterial cultivation include a soil extract medium such as SESOM, DR2A + supplements, and R2A solidified with agar or gellan (Tamaki et al., 2005; Vilain et al., 2006).
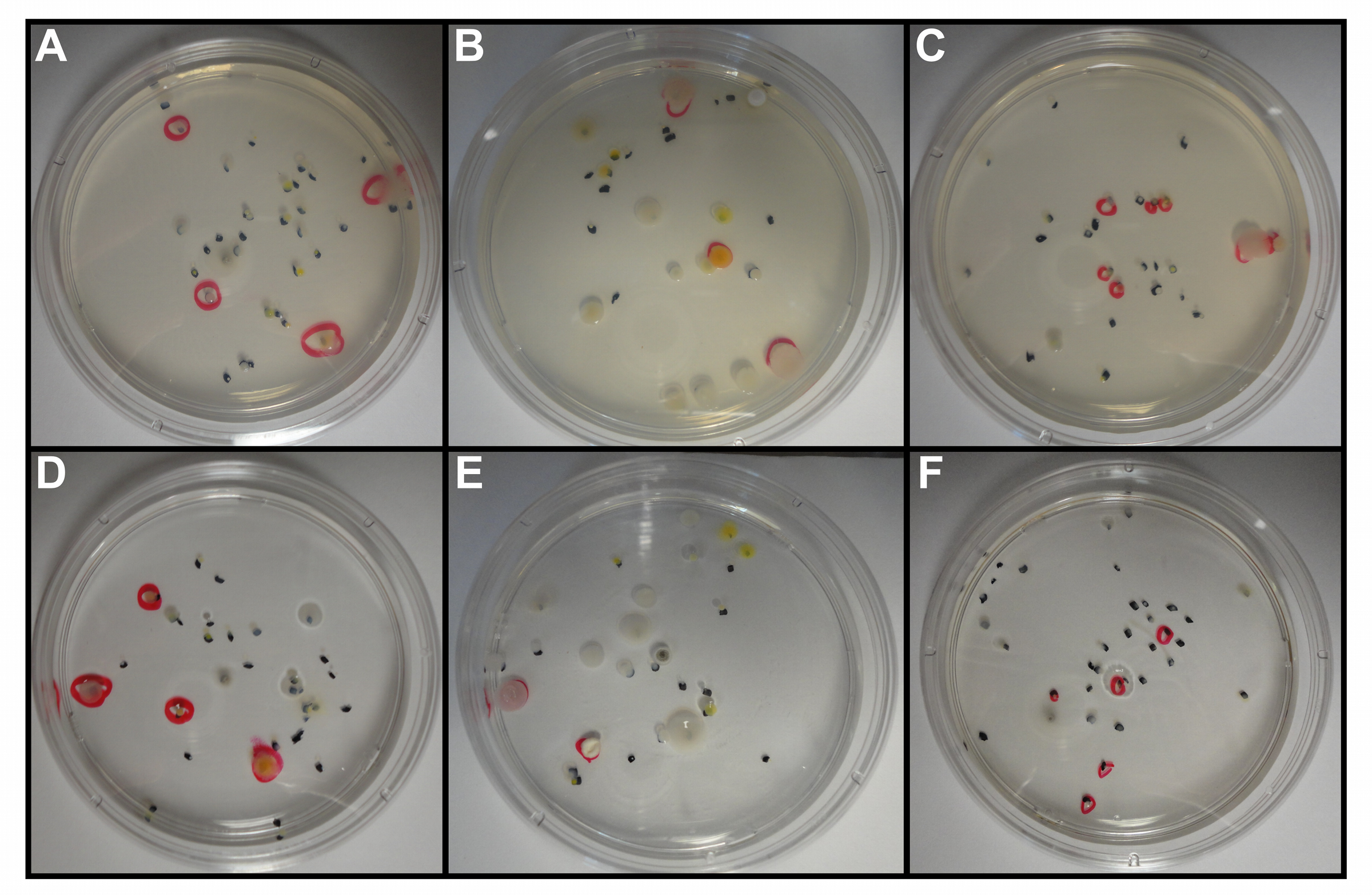
Figure 5. Bacterial cultivation of proximal soil samples from untransformed soybean roots on nutrient media solidified with agar (A-C) or gellan (D-F). Nutrient media consisted of DR2A+ (A, D), R2A (B, E), and SESOM (C, F). Bacterial samples acquired from a 10-5 dilution. Black dots and red circles indicate the presence of individual bacterial colonies. - Distal, middle, and proximal soil samples are all useful for bacterial cultivation. However, proximal soil samples are preferable as they contain the bacteria that most likely affect the plant directly and vice versa.
- After securing the lids on all the centrifuge tubes, place them into a 4 °C centrifuge and subject them to centrifugation at 5,000 x g for 10 min or 4,500 x g for 15 min (depending on the limits of the centrifuge).
- Once centrifugation is complete, discard supernatant and either immediately use the pellets for DNA or RNA isolation or store them at -80 °C until needed.
Limitations of the method
- Sonication times may vary depending on the types of plant roots used as well as the properties of the soil in which they were grown.
- It is uncertain how useful this procedure is for soil fungi.
- Sample sizes will be small (likely < 0.3 g when using 15 ml centrifuge tubes) and decrease from sonication to sonication, with proximal soil samples being the smallest. This might be an issue for methods such as proteomics and metabolomics that generally require a larger sample size.
- Age of the plant makes a difference (root system is very large at later stages). This procedure is better suited for smaller root sizes. For perennial plants or older plants with large root systems, one can use a golf cup cutter (4” to 8” diameter) to obtain a soil core (6” to 12” deep) and obtain root segments from that by placing it in water and allowing the soil to separate from the roots. Obviously, this would depend on whether the representative samples of the root system would suffice to answer the research question.
Recipes
- Phosphate buffered saline Tween 20 (PBST) (500 ml, pH of 7.2)
- Add 0.605 g of K2HPO4 to 300 ml of dH2O, stir until K2HPO4 is completely dissolved
- Add 0.17 g of KH2PO4 to mixture, stir until KH2PO4 is completely dissolved
- Add 4.1 g of NaCl to mixture, stir until NaCl is completely dissolved
- Adjust pH with NaOH or HCl until final pH is 7.2
- Add dH2O to mixture until the final volume is 500 ml, stir to ensure even distribution
- Sterilize solution via autoclaving (liquid cycle, 121 °C for 30 min)
- Add 250 µl of Tween20 to mixture, gently swirl to ensure even distribution
Note: Adding Tween20 before autoclaving will result in frothing overflow due to bubble formation. - Store at room temperature (~20 °C)
- Add 0.605 g of K2HPO4 to 300 ml of dH2O, stir until K2HPO4 is completely dissolved
Acknowledgments
This protocol was established in a previously published study (White et al., 2014). Funding for this research was provided by the South Dakota Agricultural Experiment Station and the South Dakota Soybean Research and Promotion Council. We would also like to thank R. Gelderman (SDSU) for providing soil samples, M. Hildreth (SDSU) for providing the sonicator used for this research, and Al Miron for providing the soybean plant depicted in Figure 1.
References
- Bulgarelli, D., Rott, M., Schlaeppi, K., Ver Loren van Themaat, E., Ahmadinejad, N., Assenza, F., Rauf, P., Huettel, B., Reinhardt, R., Schmelzer, E., Peplies, J., Gloeckner, F. O., Amann, R., Eickhorst, T. and Schulze-Lefert, P. (2012). Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488(7409): 91-95.
- Clark, F. E. (1940). Notes on types of Bacteria associated with plant roots. Transactions of the Kansas Academy of Science (1903-) 43:75-84.
- Doi T, H. Y., Abe, J. and Morita, S. (2007). Analysis of rhizosphere bacteria of rice cultivated in Andosol lowland and upland fields using molecular biological methods. Plant Root 1:66-74.
- Lundberg, D. S., Lebeis, S. L., Paredes, S. H., Yourstone, S., Gehring, J., Malfatti, S., Tremblay, J., Engelbrektson, A., Kunin, V., del Rio, T. G., Edgar, R. C., Eickhorst, T., Ley, R. E., Hugenholtz, P., Tringe, S. G. and Dangl, J. L. (2012). Defining the core Arabidopsis thaliana root microbiome. Nature 488(7409): 86-90.
- Philippot, L., Raaijmakers, J. M., Lemanceau, P. and van der Putten, W. H. (2013). Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11(11): 789-799.
- Tamaki, H., Sekiguchi, Y., Hanada, S., Nakamura, K., Nomura, N., Matsumura, M. and Kamagata, Y. (2005). Comparative analysis of bacterial diversity in freshwater sediment of a shallow eutrophic lake by molecular and improved cultivation-based techniques. Appl Environ Microbiol 71(4): 2162-2169.
- Vilain, S., Luo, Y., Hildreth, M. B. and Brozel, V. S. (2006). Analysis of the life cycle of the soil saprophyte Bacillus cereus in liquid soil extract and in soil. Appl Environ Microbiol 72(7): 4970-4977.
- White, L. J., Jothibasu, K., Reese, R. N., Brozel, V. S. and Subramanian, S. (2015). Spatio temporal influence of isoflavonoids on bacterial diversity in the soybean rhizosphere. Mol Plant Microbe Interact 28(1): 22-29.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
White, L. J., Brözel, V. S. and Subramanian, S. (2015). Isolation of Rhizosphere Bacterial Communities from Soil. Bio-protocol 5(16): e1569. DOI: 10.21769/BioProtoc.1569.
Category
Microbiology > Microbial cell biology > Cell isolation and culture
Microbiology > Microbe-host interactions > Bacterium
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











