- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Adoptive Transfer of Memory B Cells
Published: Vol 5, Iss 16, Aug 20, 2015 DOI: 10.21769/BioProtoc.1563 Views: 11365
Reviewed by: Achille BroggiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Expansion and Polarization of Human γδT17 Cells in vitro from Peripheral Blood Mononuclear Cells
Xu Chen [...] Jun Yan
Jan 5, 2024 1951 Views

Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
Jun 5, 2025 2620 Views

Isolation and Ex Vivo Testing of CD8+ T-Cell Division and Activation Using Mouse Splenocytes
Melissa Dolan [...] John M.L. Ebos
Aug 20, 2025 3878 Views
Abstract
The adoptive transfer of antigen-specific B cells into mice that cannot recognize that specific antigen has two main advantages. The first is determining exactly when the B cells were transferred and exposed to antigen. The second is that all B cells that can bind that antigen are the ones that were transferred; no new antigen-specific B cells will emerge from the bone marrow. Thus all B cells that were exposed to the antigen and still alive after at least 4 weeks (8 weeks or more is ideal), are memory B cells.
Splenic B cells from B1-8 mice were prepared with an EasySep Mouse B Cell Enrichment Kit according to the manufacturer’s protocol. Single-cell suspensions were transferred intravenously into tail veins of recipient mice. Approximately 1 million NP+ B cells were transferred per mouse. Approximately 12-24 h after transfer, mice were immunized intra-peritoneally with 50 µg of NP-CGG precipitated in alum.
Materials and Reagents
- Mice
Any donor mice can be used, as long as the donor and recipients have the same background strain (i.e. BALB/C into BALB/c or Bl/6 into Bl/6) to prevent rejection issues. We selected transgenic donor mice that had an increased frequency of B cells specific for our antigen of interest, NP. This way we could be certain of the number of B cells specific for our antigen and these would be easy to identify by flow cytometry and elispot. However, wild-type mice will also respond to NP, just at a lower frequency.
- B1.8+/-Jκ +/- BALB/c mice
Note: B1.8 KI BALB/c mice were generated as described (Sonoda et al., 1997) and maintained on the Jκ KO strain (Chen et al., 1993) to enrich the frequency of λ+ NP-specific B cells. B1-8 KI +/+ Jκ KO -/- mice were crossed to BALB/c mice from The Jackson Laboratory (Bar Harbor, ME) to generate B1.8+/-Jκ +/- BALB/c mice, which were used for naïve controls and for transfers of NP+ B cells used to generate MBCs.
- AM14 Tg x Vκ8R KI BALB/c mice were generated as described (Shlomchik et al., 1993; Hannum et al., 1996; Prak and Weigert, 1995), which were used as recipient mice for primary immunization
Note: All mice were maintained under specific pathogen-free conditions. The Yale Institutional Animal Care and Use Committee approved all animal experiments.
- B1.8+/-Jκ +/- BALB/c mice
- Immunizations
For generating memory B cells in a primary response, mice were immunized intra-peritoneally with 50 µg of 4-hydroxy-3-nitrophenyl acetyl (NP)-Chicken Gamma Globulin (CGG) precipitated in alum. The ratio of NP to CGG ranged between 26 and 33. All mice were immunized at 6-12 week of age
- Isolation of B cells from donor mice
- 2 pairs sterile scissor and forceps
- Sterile frosted slides
- Sterile Petri dishes
- Autoclaved Pasteur pipettes
- 70% ethanol
- Sterile ACK (RBC lysing buffer) (Lonza, catalog number: 10-548E )
- 100 µM filter (BD Biosciences, catalog number: 340615 )
- Ice
- Conical tubes (14 ml v-bottom) (BD Biosciences, Falcon®)
- Falcon 14 ml polystyrene round-bottom tubes (BD Biosciences, catalog number: 352057 )
- Trypan blue solution (0.4%) (Life Technologies, catalog number: 15250-061 )
- EasySep™ Mouse B Cell Enrichment Kit (STEMCELL Technologies, catalog number: 19754 ). Components of kit:
- EasySep™ (Negative Selection) Mouse B Enrichment Cocktail, 0.5 ml
- EasySep™ Biotin Selection Cocktail, 1 ml (store at 4 °C)
- EasySep™ Magnetic Particles, 1 ml (store at 4 °C; turn centrifuge on and cool to 4 °C)
- Normal Rat serum, 1 ml (store at -20 °C)
- EasySep™ (Negative Selection) Mouse B Enrichment Cocktail, 0.5 ml
- RPMI-1640 with L-glutamine (Sigma-Aldrich, catalog number: R8758 )
- Fetal Calf Serum (GE Healthcare HyCloneTM)
- HEPES 1 M (Corning Incorporated, catalog number: 25-060-Cl )
- Streptomycin/penicillin, 10,000 U/ml (Life Technologies, Gibco®, Catalog number 15140-122 )
- 2-mercaptoethanol (Sigma-Aldrich, catalog number: M3128 )
- PBS without Ca2+/Mg2+ (Life Technologies, Gibco®, catalog number: 10010-023 )
- Ethylenediaminetetraacetic Acid (EDTA) 0.5 M Solution (pH 8) (Thermo Fisher Scientific, catalog number: 25783 )
- 2.5% Anticoagulant citrate dextrose solution [ACD(A)] (Polymed, catalog number: 7300 )
ACDA was from the blood bank (http://www.polymedicure.com/?wpcproduct=acd-bag). Each 100 ml of ACD solution-A contains 2.2 g sodium citrate, 0.73 g citric acid, 2.45 g dextrose and 100 ml water.
- NP-binding reagents: NP-allophycocyanin (APC) (Shlomchik lab)
- Anti-CD4 (GK1.5) (Shlomchik lab)
- anti-Fc gamma RIII/II (2.4G2) (Shlomchik lab)
- anti-CD19 (1D3.2) (Shlomchik lab)
- 27 G needle, 1 ml syringe
- Ethidium Monoazide (EMA) 2 mg/ml (Molecular Probes)
- Complete media (see Recipes)
- EasySep media (see Recipes)
- Transfer buffer (see Recipes)
- Staining Media (see Recipes)
- 2 pairs sterile scissor and forceps
Equipment
- Sterile hood
- Refrigerated table top centrifuge
- Hemocytometer
- “EasySep” magnet (max vol 8 ml; min vol 250 µl) (STEMCELL Technologies, catalog number: 18001 )
Procedure
- Isolation of B cells
- Set the centrifuge temperature to 4 °C.
- Put one petri dish per spleen on top of ice in an ice bucket with 5 ml of complete media per dish.
- Euthanize mice.
- Take dead mice to sterile hood to dissect.
- Remove spleens. Place the spleen in the petri dish of complete media on ice.
- Grind the spleen between the frosted surfaces of the slides until the mixture is fairly uniform. (Alternatives included crushing spleens using the tip of a syringe or using other methods). Rinse the slides with complete media and transfer the remaining liquid through a filter into a 15 ml conical tube. Keep on ice while collecting other spleens.
- Centrifuge the cells at 4 °C, 400 RCF, for 10 min.
Note: This can vary between 8-15 min, depending on the centrifuge, available time, and concern for loss of cells.
- Remove tubes from centrifuge. Decant into a container with one swift motion (in the hood, to retain sterility). Resuspend pellet in remaining media after decanting by tapping.
- Add ACK (4 ml per spleen) to lyse the red blood cells. Incubate at RT for 4 min. During this incubation, remove connective tissue and membranes of lysed cells, which clump together and look like white filaments or “ghosts”, using a Pasteur pipette.
- Fill the conical tube to the top with complete media, invert to mix and centrifuge again at 4 °C, 400 RCF for 10 min.
- Vacuum up liquid (under sterile conditions) and resuspend the pellet in remaining media after decanting by tapping. Combine all spleens (keep genders separate).
- Count each sample in a hemocytometer. Collect 10 µl aliquot, make a dilution with Trypan blue 1:10 in PBS. Calculate volume needed for 100 x 106 cells/ml.
(live cells) (dilution) (104) = cells/ml; (cells/ml) (volume) = total cells
[Total cells]/[100x106 cells/ml] = volume needed for 108 cells/ml
- Resuspend to the correct volume in 95% EasySep medium and 5% rat serum at 108 cells/ml.
- Set the centrifuge temperature to 4 °C.
- EasySep B cell enrichment
Note: Follow manufacturer’s indications, which can change.
For processing 500 μl-8.0 ml of sample (< 8.0 x 108 cells)
- Save an aliquot of cells pre-depletion for FACS.
- Prepare cell suspension at 1 x 108 cells/ml in medium with 5% normal rat serum. Place cells in a 14 ml (17 x 100 mm) polystyrene tube.
- Add Negative Selection Mouse B cell Enrichment Cocktail at 50 μl/ml. Mix well and incubate on ice for 15 min.
- Add Biotin Selection Cocktail at 100 μl/ml. Mix well and incubate on ice for 15 min.
- Mix Magnetic Particles to ensure that they are in a uniform suspension by pipetting vigorously 5 times or vortexing quickly. Add the Magnetic Particles at 100 μl/ml. Mix well and incubate on ice for 5 min.
- Bring the cell suspension to a total volume of 5 ml (for < 4 x 108 cells) or 10 ml (for 4-8.5 x 108 cells) by adding medium without rat serum. Mix the cells in the tube by pipetting gently 2-3 times.
- Place the tube (without cap) into the magnet. Set aside for 5 min at room temperature.
- Pick up the magnet and in one continuous motion invert the magnet and tube, pouring off the desired fraction into a new 14 ml tube. Leave the magnet and tube in inverted position for 2-3 sec, and then return to the upright position. Do not shake or blot off any drops that may remain hanging from the mouth of the tube.
- Count cells using a hemocytometer.
- Check purity and antigen specific cell percentage by flow cytometry.
- Save at least 2 x 106 cells for staining.
- Place in 96-well plate for staining.
- Centrifuge at 280 RCF, 4 °C for 4 min.
- Prepare antibody cocktail assuming 50 μl for each sample, antibody mix: NP-APC, CD19-Pacific Blue, CD4-FITC.
- Decant residual volume in sink with a quick inversion.
- Resuspend pelleted cells in 50 μl of staining media.
- Add 50 μl of antibody cocktail to each well.
- Mix well with pipette up and down.
- Incubate for 20 min on ice, covered with aluminum foil.
- Add 100 μl of staining media.
- Centrifuge at 280 RCF, 4 °C for 4 min.
- Decant residual volume in sink with a quick inversion.
- Resuspend pelleted cells in ~175 μl of staining media.
- Centrifuge at 280 RCF, 4 °C for 4 min.
- Decant residual volume in sink with a quick inversion.
- Resuspend pellet in 175 μl of PBS.
- Transfer samples from 96-well plate into test tubes immediately before going to the flow cytometry facility.
- Add 0.02 μl of EMA per sample right before running on flow cytometer.
- Save at least 2 x 106 cells for staining.
- Save an aliquot of cells pre-depletion for FACS.
- Mouse Injection
Determine number of cells needed. We would transfer 1 million antigen-specific cells per mouse. We would determine the percentage of antigen-specific B cells in a sample by flow cytometry and then transfer the total number of cells accordingly. We also determined the percentage purity of B cells by flow cytometry. The purity of B cells was typically 90%.
Inject 1 million NP+ B viable cells suspended in transfer buffer per mouse intravenously in 0.2 ml volume.
Representative data
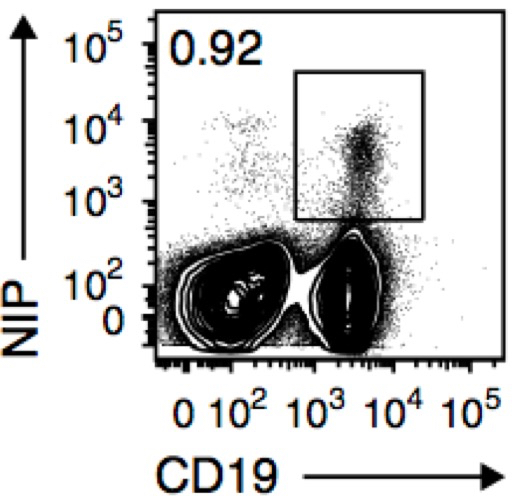
Figure 1. Flow cytometry of splenic cells from AM14-Tg x Vκ8R-KI recipient mice given NP-specific B cells and immunized with NP-CGG in alum, assessed 8 weeks later. Number adjacent to outlined area indicates percent CD19+NP+ antigen-specific B cells among live cells. (from Zuccarino-Catania et al., 2014)
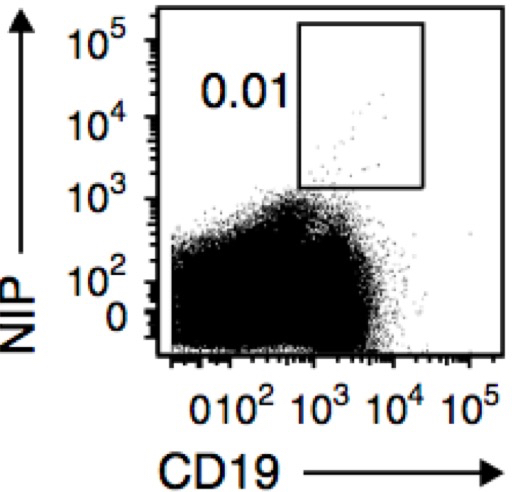
Figure 2. Flow cytometry of splenic B cells from AM14-Tg x Vκ8R-KI mice immunized with NP-CGG in alum without transfer of NP-specific B cells, assessed 8 weeks later as in Figure 1. (from Zuccarino-Catania et al., 2014)
Notes
- Usually mice are dissected in a sterile hood, so that splenic B cells remain sterile after harvesting. Cells should be kept cold at all times to minimize death and activation prior to transfer (either on ice or in 4 °C fridge).
- Another option, instead of B cell enrichment, is to do a complement depletion of T cells. This is a bit cheaper and could yield similar levels of purity. We decided to use the EasySep method from the start and got good results, so we continued with this method. It is important to use a method of enriching B cells, without their activation. We also wanted to avoid transferring T cells from our donor mice, to avoid any rejection issues (our recipients had intact naïve T cells that would help the generation of memory B cells just as well as donor T cells).
Recipes
- Complete media
443.5 ml RPMI-1640 w/L-glutamine (or add 5 ml L-glu to 500 ml RPMI)
50 ml fetal calf serum
5 ml HEPES (10 mM)
1 ml streptomycin/penicillin
0.5 ml 2-mercaptoethanol (50 mM)
Combine ingredients and filter
Keep in 4 °C fridge until needed
- EasySep media
244.5 ml1x PBS without Ca2+/Mg2+
0.5 ml0.5 M stock EDTA (final is 1 mM)
5 ml2% calf serum
- Transfer buffer
50 ml1x PBS without Ca2+/Mg2+, sterile filtered
0.5 ml 10 mM HEPES
0.25 mlstreptomycin/penicillin
1.25 ml2.5% ACDA
- Staining media
1 L1x PBS without Ca2+/Mg2+
30 mlfetal calf serum (3% final concentration)
2.5 mlNaN3 stock (0.04%)
Acknowledgments
This protocol was developed or modified in Dr. Mark Shlomchik’s laboratory at Yale University. Supported by the National Institutes of Health (R01-AI46303 to M.J.S) and NSF Graduate Research fellowships (G.V.Z.-C.). This protocol was adapted from Tomayko et al. (2010) and Sweet et al. (2013).
References
- Chen, J., Trounstine, M., Kurahara, C., Young, F., Kuo, C., Xu, Y., Loring, J., Alt, F. and Huszar, D. (1993). B cell development in mice that lack one or both immunoglobulin kappa light chain genes. EMBO J 12(3): 821.
- Hannum, L. G., Ni, D., Haberman, A. M., Weigert, M. G. and Shlomchik, M. J. (1996). A disease-related rheumatoid factor autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. J Exp Med 184(4): 1269-1278.
- Prak, E. L. and Weigert, M. (1995). Light chain replacement: a new model for antibody gene rearrangement. J Exp Med 182(2): 541-548.
- Shlomchik, M. J., Zharhary, D., Saunders, T., Camper, S. A. and Weigert, M. G. (1993). A rheumatoid factor transgenic mouse model of autoantibody regulation. Int Immunol 5(10): 1329-1341.
- Sonoda, E., Pewzner-Jung, Y., Schwers, S., Taki, S., Jung, S., Eilat, D. and Rajewsky, K. (1997). B cell development under the condition of allelic inclusion. Immunity 6(3): 225-233.
- Sweet, R. A., Cullen, J. L. and Shlomchik, M. J. (2013). Rheumatoid factor B cell memory leads to rapid, switched antibody-forming cell responses. J Immunol 190(5): 1974-1981.
- Tomayko, M. M., Steinel, N. C., Anderson, S. M. and Shlomchik, M. J. (2010). Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol 185(12): 7146-7150.
- Zuccarino-Catania, G. V., Sadanand, S., Weisel, F. J., Tomayko, M. M., Meng, H., Kleinstein, S. H., Good-Jacobson, K. L. and Shlomchik, M. J. (2014). CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol 15(7): 631-637.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zuccarino-Catania, G. and Shlomchik, M. (2015). Adoptive Transfer of Memory B Cells. Bio-protocol 5(16): e1563. DOI: 10.21769/BioProtoc.1563.
Category
Immunology > Immune cell isolation > Lymphocyte
Immunology > Immune cell function > Antigen-specific response
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










