- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Animal Models of Corneal Injury
Published: Vol 5, Iss 13, Jul 5, 2015 DOI: 10.21769/BioProtoc.1516 Views: 11051
Reviewed by: Jia LiAndrea IntroiniAlka Mehra

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2516 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2539 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3975 Views
Abstract
The cornea is an excellent model system to use for the analysis of wound repair because of its accessibility, lack of vascularization, and simple anatomy. Corneal injuries may involve only the superficial epithelial layer or may penetrate deeper to involve both the epithelial and stromal layers. Here we describe two well-established in vivo corneal wound models: a mechanical wound model that allows for the study of re-epithelialization and a chemical wound model that may be used to study stromal activation in response to injury (Stepp et al., 2014; Carlson et al., 2003).
Keywords: CorneaMaterials and Reagents
Note: All reagents may be maintained at room temperature.
- FVB mouse (5-10 weeks old)
- Isoflurane (Abbott Laboratories, catalog number: 5260-04-05 )
- Proparacaine hydrochloride ophthalmic solution (0.5%) (Bausch & Lomb, NDC: 24208-730- 06 )
- Fluorescein sodium and benoxinate hydrochloride ophthalmic solution (0.25%/0.4%) (Akorn, NDC: 17478-640-10 )
- Weck-cel cellulose eye spears (Medtronic, catalog number: 0008680 )
- NaOH solution (see Recipes)
Chemical injury
- Sterile water
- 1x sterile phosphate buffered saline buffer (1x PBS)
- Sodium hydroxide (NaOH) 1.0 N (normal) solution (Sigma-Aldrich, catalog number: S2770 )
- Fluorescein solution (see Recipes)
Equipment
- Heating pad for mouse
- Algerbrush II with 0.5 mm Burr (Katena, catalog number: K 2-4900 )
- Trephine with handle (1.5 mm) (Beaver-visitec, catalog number: 9748 )
- Alcohol swabs - isopropyl alcohol 70% (BD, catalog number: 326895 )
- Filter paper (Thermo Fisher Scientific, catalog number: 09-795AA )
- Ear punch (Roboz, catalog number: 65-9902 )
- Forceps (Dumont #5)
- Pipette with tips (1 ml)
- Timer
- Stereo microscope (for scratch/epithelial injury, need filter set to visualize GFP; for both injuries, camera attachment is optional) (Leica, catalog number: MZ16F )
- Anesthesia machine with nose cone attachment appropriate for mice (Summit Anesthesia Solutions, catalog number: AS-01-0007 )
Procedure
Ethical statement: All procedures discussed here are in accordance with and were approved by the University of California, San Francisco Institutional Animal Care and Use Committee.
For scratch/epithelial injury
- Set up the surgical area.
- Place the isoflurane chamber near the microscope and place the anesthesia platform with the nose cone under the microscope objective. Place a mouse heating pad by the nose cone.
- Place the Proparacaine, Algerbrush, weck-cels, and trephine on the lab bench near the surgical area. Have 20 μl of 1:40 diluted Fluorescein solution drawn up in a pipette (see Recipes).
- Clean off the Algerbrush burr by rubbing with an alcohol swab.
- Place the isoflurane chamber near the microscope and place the anesthesia platform with the nose cone under the microscope objective. Place a mouse heating pad by the nose cone.
- Anesthetize the mouse in the isoflurane chamber. A typical approach for anesthesia involves placing the animal in an induction chamber connected to an oxygen source and isoflurane vaporizer, and adjusting oxygen flow to 0.9 liters/min and the isoflurane vaporizer to 1-2%. As soon as the mouse becomes unresponsive and has shallow breathing, it may be transferred to the anesthesia platform with the nose cone and placed on a heating pad. Position the mouse head so that the eye to be injured is facing up towards the microscope objective.
- Squeeze the bottle of Proparacaine and place 1 drop of Proparacaine on the cornea. Wait 30 sec.
- Use a weck-cel to dry off the cornea by gently sweeping across cornea once and dabbing both corners of the eye.
- Apply periocular pressure with one hand to proptose the mouse eye. (Optional step: Take a brightfield picture of the cornea prior to injury.)
- Use the other hand to mark the cornea with the trephine as central as possible with gentle pressure. Hold the handle of trephine with your thumb and second fingers and place the entire circular edge on the cornea. Gently turn the trephine with mild pressure approximately 3 clock hours to mark the cornea. Avoid making multiple marks.
- Turn the Algerbrush on and make an epithelial defect in the center of the cornea by applying gentle pressure in a circular manner and observing a break in the surface epithelial cell layer. Carefully extend the epithelial defect close to the trephine mark.
- Turn the Algerbrush off and use the dull blades of the Algerbrush burr to gently remove the remaining corneal epithelium out to the trephine mark. Apply Fluorescein solution to the cornea and confirm size of epithelial defect using the microscope GFP filter.
- Optional step: Apply periocular pressure to proptose the mouse eye. Take a fluorescence picture of the wounded cornea after applying Fluorescein solution and using the GFP filter (Figure 1).
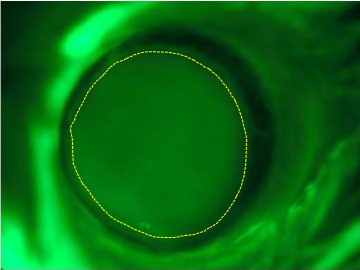
Figure 1. Corneal scratch/epithelial injury. Photograph of an epithelial defect of the central cornea after scratch injury stained with Fluorescein solution. The border of the scratch wound is in shown in yellow. [adapted from Figure 2 of Chan et al. (2013)]
- Place 1 drop of Proparacaine on the cornea.
- Remove mouse from the nose cone and allow mouse to awaken in a recovery cage. Monitor the mouse for pain and eye infections.
For chemical injury
- Set up the surgical area.
- Place the isoflurane chamber near the microscope and place the anesthesia platform with the nose cone under the microscope objective.
- Place the Proparacaine, filter paper, forceps, weck-cels, and NaOH on the lab bench near the surgical area.
- Have the pipette with 500 μl PBS drawn up readily available. (Optional: Have 20 μl Fluorescein solution drawn up in a pipette.)
- Prepare 2 mm filter paper discs using the ear punch.

Figure 2. Filter paper disc. An ear punch can be used to create uniform 2 mm filter paper discs.
- Set the timer for 10 sec and 30 sec.
- Place the isoflurane chamber near the microscope and place the anesthesia platform with the nose cone under the microscope objective.
- Anesthetize the mouse in the isoflurane chamber. A typical approach for anesthesia involves placing the animal in an induction chamber connected to an oxygen source and isoflurane vaporizer, and adjusting oxygen flow to 0.9 L/min and the isoflurane vaporizer to 1-2%. As soon as the mouse becomes unresponsive and has shallow breathing, it may be transferred to the anesthesia platform with the nose cone and placed on a heating pad. Position the mouse head so that the eye to be injured is facing up towards the microscope objective.
- Place 1 drop of Proparacaine on the cornea. Wait 30 sec.
- Use a weck-cel to dry off the cornea by gently sweeping across cornea once and dabbing both corners of the eye.
- Use the forceps to submerge the filter paper into the NaOH solution for exactly 10 seconds.
- Apply periocular pressure with one hand to proptose the mouse eye. (Optional step: Take a brightfield picture of the cornea prior to injury.)
- Use forceps in the other hand to apply the NaOH-soaked filter paper to the center cornea for exactly 30 sec. Perform this step using the stereomicroscope to precisely place the filter paper as central as possible.
- Remove the filter disc from the cornea using forceps.
- Immediately flush the eye with 500 μl PBS to wash away residual NaOH (apply PBS, dry with weck-cel, apply PBS, dry with weck-cel, repeat until all 500 μl PBS has been used).
- Optional step: Apply periocular pressure to proptose the mouse eye. Take a fluorescence picture of the wounded cornea after applying Fluorescein solution and using the GFP filter.
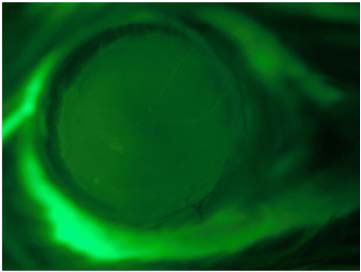
Figure 3. Corneal chemical injury. Photograph of an epithelial defect of the central cornea after chemical injury stained with Fluorescein solution [adapted from Figure 1 of Chan et al. (2013)]
- Place 1 drop of Proparacaine on the cornea.
- Remove mouse from the nose cone and allow mouse to awaken in a recovery cage. Monitor mouse for pain and eye infections.
Notes
- When using the scratch injury model to compare epithelial repair rates between mice, it is recommended that littermate and/or age-matched mice are used.
Recipes
- NaOH solution
Mix the following ingredients in a 1.5 ml Eppendorf tube the same day of the procedure: 50 μl 1 N NaOH and 450 μl sterile H2O to gain a total volume of 500 μl.
- Fluorescein solution (optional for chemical injury)
Prepare the 1:40 dilution in a 1.5 ml Eppendorf tube the same day of the procedure: 2 μl Fluorescein sodium/benoxinate hydrochloride ophthalmic solution and 78 μl sterile PBS to gain a total volume of 80 μl.
Acknowledgments
This work was supported by grants from the National Institutes of Health (K08 EY018858 and R01 EY002162 to M.F.C. and R01 CA057621and P01 AI053194 to Z.W.).
References
- Carlson, E. C., Wang, I. J., Liu, C. Y., Brannan, P., Kao, C. W. and Kao, W. W. (2003). Altered KSPG expression by keratocytes following corneal injury. Mol Vis 9: 615-623.
- Chan, M. F., Li, J., Bertrand, A., Casbon, A. J., Lin, J. H., Maltseva, I. and Werb, Z. (2013). Protective effects of matrix metalloproteinase-12 following corneal injury. J Cell Sci 126(Pt 17): 3948-3960.
- Stepp, M. A., Zieske, J. D., Trinkaus-Randall, V., Kyne, B. M., Pal-Ghosh, S., Tadvalkar, G. and Pajoohesh-Ganji, A. (2014). Wounding the cornea to learn how it heals. Exp Eye Res 121: 178-193.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chan, M. F. and Werb, Z. (2015). Animal Models of Corneal Injury. Bio-protocol 5(13): e1516. DOI: 10.21769/BioProtoc.1516.
Category
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link