- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Visual Assessment of the Severity of Fusarium Seedling Blight (FSB) and Fusarium Head Blight (FHB) Disease in Barley
Published: Vol 5, Iss 12, Jun 20, 2015 DOI: 10.21769/BioProtoc.1507 Views: 10358
Reviewed by: Samik BhattacharyaPablo Bolanos-VillegasSollapura J. Vishwanath

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An Assay to Study Botrytis cinerea-infected Grapevine Leaves Primed with Pseudomonas fluorescens
Charlotte Gruau [...] Aziz Aziz
Oct 5, 2016 12843 Views

Assessment of Wheat Resistance to Fusarium graminearum by Automated Image Analysis of Detached Leaves Assay
Alexandre Perochon and Fiona M. Doohan
Dec 20, 2016 12751 Views

Quantification of the Composition Dynamics of a Maize Root-associated Simplified Bacterial Community and Evaluation of Its Biological Control Effect
Ben Niu and Roberto Kolter
Jun 20, 2018 10502 Views
Abstract
Fusarium pathogens are among the most damaging pathogens of cereals. These pathogens have the ability to attack the roots, seedlings, and flowering heads of barley and wheat plants (Simpson et al., 2004). Resulting in yield loss and head blight disease and also resulting in the contamination of grain with mycotoxins harmful to human and animal health (McMulen et al., 1997; Walter et al., 2010; Agostinelli et al., 2012). The study of Fusarium diseases, including host disease resistance and the effect of exogenous agents (chemicals, biocontrol agents, etc.), requires robust and effective methods for the assessment and quantification of visual disease symptoms. Here we describe the methods commonly used for the assessment and quantification of the severity of Fusarium seedling blight and Fusarium head blight disease.
Materials and Reagents
- Fusarium culmorum strain FCF 200 (Courtesy of Paul Nicholson, https://www.jic.ac.uk/directory/paul-nicholson/)
- Potato dextrose agar (Difco, catalog number: 213400 )
- Mung bean (any grocery store)
- John Innes compost No 2 (Westland Horticulture)
- Tween 20 (Sigma-Aldrich, catalog number: P2287 )
- Fertiliser NPK 10-10-20 (Agrifert)
- Agar (OXOID, catalog number: LP0013 )
- Filter paper (Whatman, catalog number: 1001-090 )
- Mung bean broth (see Recipes)
Equipment
- KOVA Glasstic Slide 10 (KOVA International Inc., catalog number: 87144 )
- Silicon tubing (0.5 cm diameter)
- Plant growth room
- -70 °C freezer (Thermo Scientific Revco PLUS)
- Shaking incubator (New Brunswick Scientific, model: I26 )
- Light microscope (Leica Microsystems, model: DM3000 )
- Glasshouse (CambridgeHOK containment glasshouse) [22 °C; maximum temperature of 27 °C and minimum light intensity of 700 μmol/m2/s under a 16 h/8 h day (700 μmol/m/s)/night regime or a contained environment room with an optimal 16 h/8 h day (700 μmol/m/s)/night at 20/12 °C]
Procedure
- Preparation of fungal conidial inoculum
- Store F. culmorum mycelia at -70 °C in 10% (vv-1) glycerol. Prior to use, culture the Fusarium isolate on potato dextrose agar (PDA) plates and incubated at 25 °C for 7 days.
- For conidial production, inoculate 3-4 PDA-agar plugs in 100 ml conical flask containing 25 ml Mung bean broth media (see Recipes) and incubate cultures at 200 rpm, and at 25 °C for 5 days without light.
- Pass the conidial suspension through two layers of sterile cheese cloth and wash three times with sterile distilled water. Resuspend conidia in 0.2% Tween 20 and determine the concentration using disposable KOVA Glasstic Slides 10. Adjust the concentration to 2 x 106 spores/ml 0.2% Tween 20.
Note: For spore counting using KOVA Glasstic slides, determine the average number of spores per small grid and multiply with 90,000 to get number spores/ml.
- Store F. culmorum mycelia at -70 °C in 10% (vv-1) glycerol. Prior to use, culture the Fusarium isolate on potato dextrose agar (PDA) plates and incubated at 25 °C for 7 days.
- Fusarium seedling blight
- Place barley seeds in a 9 cm petri plate containing 2 pieces of filter paper (90 mm diameter) soaked with 6ml of sterile distilled water.
- Cover the petri plates with aluminum foil and stratify in the dark for 2 days at 4 °C.
- Transfer plates to 21 °C in the dark for 2 days to facilitate germination.
- Transfer germlings to a 6 cm-diameter pot containing John Innes compost No 2 and place a 2-cm length of sterile silicon tubing (0.5-cm-diam.) as a collar around the stem base.
- Place plants in a climate-controlled growth room at 22 °C under long day conditions (16 h/8 h), bottom watering every second day. Relative humidity was maintained at 70%.
- Inoculate the stem bases of 10-day-old seedlings with a 400 μl F. culmorum conidial suspension [1 x 106 spores/ml 0.2% Tween 20 in 1% (wv-1) agar]. Treat the mock control plants with 1% agar containing 0.2% Tween 20.
Note: Prepare 2% (wv-1) sterile agar solution and bring it to 50 °C. Mix the spore suspension (2 x 106 spores/ml) and the agar solution at a 1:1 ratio. - Score visible disease symptoms (Figure 1) on the stems at 12 days post-inoculation using the following disease scoring system: FSB disease scores were the product of lesion length (cm) by lesion colour (lesion colour scale: 0, no disease; 1, very slight brown necrosis; 2, slight/moderate brown necrosis; 3, extensive brown necrosis; 4, extensive black necrosis) (Nicholson et al., 1998).
- Repeat the experiment three times, each time including three replicate pots (each containing two plants) per treatment combination in a randomised layout.

Figure 1. Barley seedlings displaying symptoms of Fusarium (cultivar Lux). Ten-day-old seedlings were treated with: A 0.2% Tween 20 and 1% (wv-1) agar, or B conidia of Fusarium culmorum (strain FCF 200) in 0.2% Tween 20 and 1% agar. Disease symptoms (browning of the stem base) were visualised 12 days post-treatment.
- Place barley seeds in a 9 cm petri plate containing 2 pieces of filter paper (90 mm diameter) soaked with 6ml of sterile distilled water.
- Fusarium head blight
- Germinate the barley seeds as described above for the Fusarium seedling blight experiments.
- Transfer germinated seed to a 15 cm-diameter pot containing John Innes compost No 2. Cultivate plants in the glasshouse. Add 2 g of NPK 10-10-20 fertiliser to each pot at growth stages (GS; Zadoks et al., 1974) 20 and 30.
Note: For more information on different Zadoks growth stages go to http://www.cerealcentral.ca/crop-management_cereal-staging.aspx. - Treat the heads at mid-anthesis (GS 65) by injecting 4 µl of conidial suspension (5x 104 spores/ml 0.2% Tween 20) or mock 0.2% Tween 20 treatment into each of the four middle spikelets of a head.
- Immediately cover treated heads with a polythene bag for 48 h in order to promote Fusarium infection.
- Score the FHB disease symptoms (Figure 2) (percentages bleached spikelets per head) at GS 70, 80 and 90 and calculate the Area Under the Disease Progress Curve (AUDPC) [as described by Shaner and Finney (1977)].
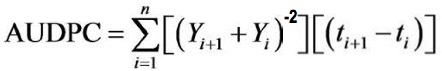
Where, Yi is an assessment of a disease progression (percentages bleached spikelets per head) at the ith observation, ti is time (day) at the ith observation, and n is the total number of observations (Shaner and Finney, 1977). - When ripe (GS 91), harvest the cereal heads, freeze-dry for 48 h and determine the number and weight of grains in each head. Use this data to calculate the 1,000 grain weight.
- The glasshouse FHB disease trials should be conducted at least twice and each trial should include at least 5 replicate plants (2 heads per plant) per treatment combination arranged in a randomised layout.

Figure 2. Symptoms of Fusarium head blight in barley (cultivar Akashinriki). Four middle spikelets were injected with 4 µl of A conidia suspension of Fusarium culmorum (strain FCF 200) in 0.2% Tween 20, or B 0.2% Tween 20. Disease symptoms (percentages of bleached spikelets per head) and AUDPC were scored at GS 80. White arrow indicates the point of inoculation.
- Germinate the barley seeds as described above for the Fusarium seedling blight experiments.
Recipes
- Mung bean broth
Boil 20 g/L mung bean in distilled water for 20 min
Pass the broth through two layers of cheese cloths and make up the volume up to 1 L
Autoclave for 15-20 min at 121 °C
Acknowledgments
This work was supported by the Science Foundation Ireland research fund (IN10/IN.1/B3028) and Department of Agriculture Research Stimulus Grant RSF 07 513.
References
- Agostinelli, A. M., Clark, A. J., Brown-Guedira, G. and Van Sanford, D. A. (2012). Optimizing phenotypic and genotypic selection for Fusarium head blight resistance in wheat. Euphytica 186(1): 115-126.
- Ali, S. S., Gunupuru, L. R., Kumar, G. B., Khan, M., Scofield, S., Nicholson, P. and Doohan, F. M. (2014). Plant disease resistance is augmented in uzu barley lines modified in the brassinosteroid receptor BRI1. BMC Plant Biol 14: 227.
- Ali, S. S., Kumar, G. B., Khan, M. and Doohan, F. M. (2013). Brassinosteroid enhances resistance to fusarium diseases of barley. Phytopathology 103(12): 1260-1267.
- McMullen, M., Jones, R. and Gallenberg, D. (1997). Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis 81(12): 1340-1348.
- Nicholson, P., Simpson, D., Weston, G., Rezanoor, H., Lees, A., Parry, D. and Joyce, D. (1998). Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol Mol Plant Pathol 53(1): 17-37.
- Shaner, G. and Finney, R. (1977). The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology 67(8): 1051-1056.
- Simpson, D. R., Thomsett, M. A. and Nicholson, P. (2004). Competitive interactions between Microdochium nivale var. majus, M. nivale var. nivale and Fusarium culmorum in planta and in vitro. Environ Microbiol 6(1): 79-87.
- Walter, S., Nicholson, P. and Doohan, F. M. (2010). Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol 185(1): 54-66.
- Zadoks J. C., Chang T. T. and Koznak C .F. (1974). A decimal code for the growth stages of cereals. Weed Res 14(6): 415-421.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ali, S. S., Gunupuru, L. R. and Doohan, F. M. (2015). Visual Assessment of the Severity of Fusarium Seedling Blight (FSB) and Fusarium Head Blight (FHB) Disease in Barley. Bio-protocol 5(12): e1507. DOI: 10.21769/BioProtoc.1507.
Category
Microbiology > Microbe-host interactions > In vivo model > Plant
Microbiology > Microbe-host interactions > Fungus
Plant Science > Plant immunity > Disease bioassay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










