- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Two-event Transfusion-related Acute Lung Injury Mouse Model
Published: Vol 5, Iss 12, Jun 20, 2015 DOI: 10.21769/BioProtoc.1505 Views: 9644
Reviewed by: Andrea PuharMaureen WirschellAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visualizing Hypoxia in a Murine Model of Candida albicans Infection Using in vivo Biofluorencence
José Pedro Lopes and Constantin F. Urban
Aug 5, 2019 5112 Views

RNA Extraction from Ears and Draining Lymph Nodes of Mice Infected with Leishmania amazonensis
Emilie Giraud and Evie Melanitou
Jun 5, 2020 5516 Views

TetR Regulated in vivo Repression Technology to Identify Conditional Gene Silencing in Genetically Engineerable Bacteria Using Vibrio cholerae Murine Infections as Model System
Franz G. Zingl [...] Stefan Schild
Oct 5, 2020 3749 Views
Abstract
Transfusion-related acute lung injury (TRALI) is defined as acute lung injury that occurs within 6 hours of a blood product transfusion. TRALI continues to be a leading cause of transfusion-related mortality and we have developed a mouse model of TRALI to better understand the mechanisms by which injury occurs and to test therapeutic approaches. Our model is a two-event model based on immune priming and the challenge of BALB/c wild-type mice with cognate MHC Class I monoclonal antibody (MHC I mAb). Immune priming with LPS mimics the primed state of recipients (first event) that is important for the development of TRALI. Donor HLA antibodies are frequently implicated in TRALI reactions, and cognate MHC Class I antibody (second event) produces acute lung injury in primed animals. Here, we describe a detailed protocol with high reproducibility within animals.
Keywords: TransfusionsMaterials and Reagents
- BALB/c WT mice (male, age 8-12 weeks)
- MHC Class I mAb (H2Kd, IgG2a,κ; stock concentration 0.65 mg/ml) is purified from a hybridoma (ATCC, catalog number: 34-1-2S ) using standard protein A affinity chromatography.
- Isotype-matched mAb (IgG2a,κ, stock concentration 1 mg/ml) (BD, catalog number: 553453 )
- LPS (Sigma-Aldrich, catalog number: L2880 )
- Ketamine (Henry Schein, catalog number: 010177 ) and Xylazine (Henry Schein, catalog number: 033198 ) for mouse anesthesia
- Heparin Sodium Injection (Sagent Pharmaceuticals, NDC number: 25021 )
- Phosphate buffered saline (PBS) (see Recipes)
Equipment
- Surgical scissors
- Curved blunt-ended forceps
- 25 gauge and 28G sterile needles
- Insulin syringe (0.5 ml) and attached needle (sterile)
- 1 ml sterile syringes
- 30-gauge sterile needle for jugular vein cannulation
- Polyethylene tubing PE-10 (Intramedic, catalog number: 427401 )
- Cotton tipped applicator
- 6-0 silk suture
- Warming pad
- Tape
Procedure
- Preparation
All protocols using live animals must be approved by an Institutional Animal Care and Use Committee and must follow officially approved procedures for the care and use of laboratory animals. Furthermore, experimental animals treated with biohazard materials should be handled and disposed using recommended animal biosafety procedures. When appropriate, the person(s) handling the animals must have official certification for performing procedures on animals and for submitting protocols for ethical approval.
- Weigh the animal on a balance (grams) in order to calculate the LPS dose required for each mouse.
- Prepare LPS stock solution by diluting LPS with sterile water to a concentration of 0.1 mg/ml. Aliquot (500 µl) and store at -80 °C.
- Desired LPS priming dose = 0.1 mg/kg. From the body weight of the mouse (measured in grams), convert this number to µl. Pipette this amount from the LPS stock solution to an Eppendorf tube and dilute to 150 μl final volume with sterile PBS. For example, for a 25 gram mouse, add 25 µl of LPS stock solution (0.1 mg/ml) to an Eppendorf tube and dilute to 150 µl final volume.
- Prepare MHC Class I mAb or isotype control mAb (0.5-4.5 mg/kg) and dilute to 100 μl final volume.
- Prepare a sterile heparin solution (4 units/ml) to flush the PE-10 tubing.
- Load insulin syringe with attached needle with sterile heparin solution (4 units/ml) and insert needle into PE-10 tubing (6 inches; 15 cm) on one end, and attach a sterile 30 gauge needle on the opposite end (Figure 1).
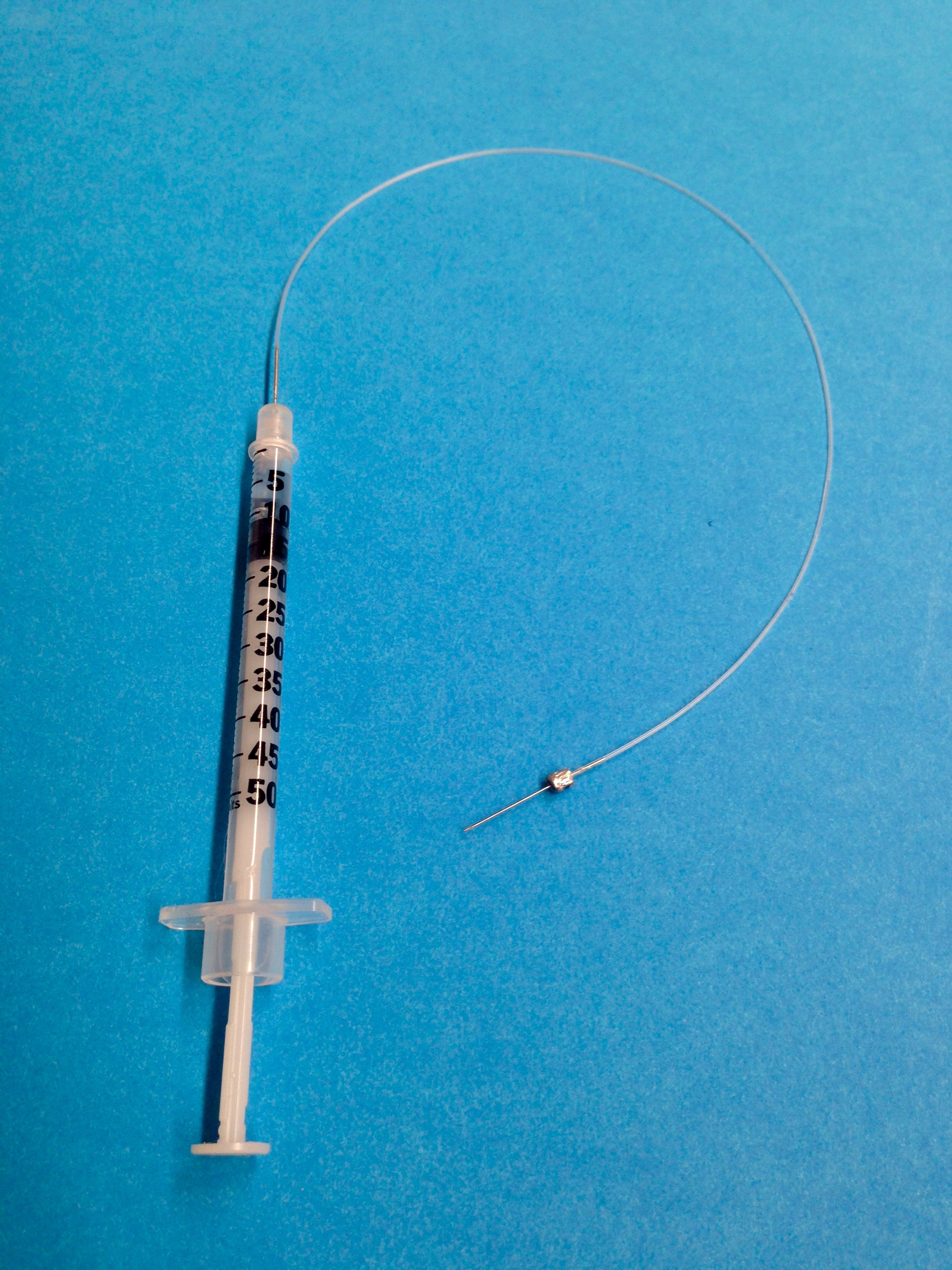
Figure 1. Representative setup for jugular vein cannulation
- Weigh the animal on a balance (grams) in order to calculate the LPS dose required for each mouse.
- Transfusion-related acute lung injury model
First event: immune priming with LPS
Immune priming is necessary to produce robust lung injury when using mice housed in pathogen-free barrier rooms (Looney et al., 2009).
- Twenty-four hours prior to challenge with MHC I mAb mice, inject mice with LPS (0.1 mg/kg, i.p.).
All surgical procedures should be performed using standardized aseptic techniques and all surgical tools should be autoclaved following the surgery.
- Anesthetize animals with Ketamine (50-80 mg/kg) and Xylazine (8-12 mg/kg) injected intraperitoneal using insulin syringes. The anesthetics may be mixed in the same syringe.
- Place the mouse on a warming pad. Check the level of anesthesia using a paw pinch stimulus a few minutes after delivery of the anesthetics. Once adequate anesthesia is observed, immobilize the mouse in the supine position using adhesive tape.
- Make a vertical cut (~0.5 cm) to the right of the trachea and isolate the right jugular vein (Figure 2A).
- Carefully introduce the needle into the vessel. The needle, syringe, and PE-10 tubing should be filled with a heparin solution (4 units/ml). Aspirate blood from the jugular vein to verify intravascular placement of the needle (Figure 2B).
- Remove the syringe with heparin solution and replace with an insulin syringe (with attached needle) that has been preloaded with the MHC Class I mAb vs. isotype control mAb.
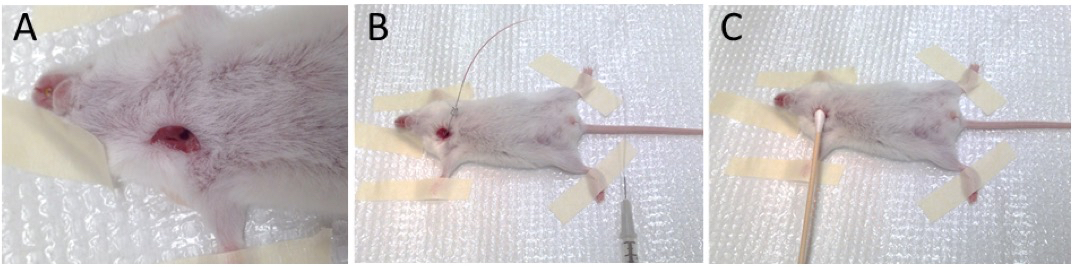
Figure 2. Isolation of right jugular vein (A), cannulation and aspiration of blood (B), and removal of needle and placement of cotton-tip applicator (C)
- Flush the whole syringe content at a constant and uniform rate with particular care of not introducing any air bubbles.
- Carefully remove the needle and apply a cotton-tipped applicator with slight pressure to prevent bleeding (Figure 2C). Pressure should be applied for at least 1 min.
- Close the incision with 6-0 silk suture and apply post-operative care based on the institution’s animal care guidelines. Mice should be kept on a warming pad for the duration of the experiment and closely monitored for evidence of abnormal breathing.
- Mice are killed at 2 h after injection of the mAb or when they appear moribund.
- After carefully opening the thorax, collect blood from the right ventricle using a 25 gauge needle and a 1 ml syringe.
- Remove the lungs by cutting the trachea being careful to not let pulmonary edema fluid escape.
Representative data
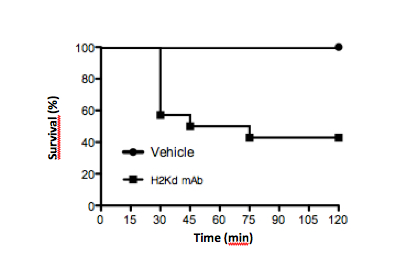
Figure 3. Typical survival curve in mice challenged with MHC Class I mAb against H2Kd (1 mg/kg, i.v.) vs. vehicle control
Recipes
- Phosphate buffered saline (PBS)
0.2g/L KH2PO4
2.16 g/L Na2HPO4
0.2 g/L KCl
8.0 g/L NaCl
Sterile filtered
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute grant R01 HL107386 (M.R.L.).
References
- Looney, M. R., Nguyen, J. X., Hu, Y., Van Ziffle, J. A., Lowell, C. A. and Matthay, M. A. (2009). Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest 119(11): 3450-3461.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ortiz-Muñoz, G. and Looney, M. R. (2015). Two-event Transfusion-related Acute Lung Injury Mouse Model. Bio-protocol 5(12): e1505. DOI: 10.21769/BioProtoc.1505.
Category
Immunology > Animal model > Mouse
Microbiology > Microbe-host interactions > In vivo model > Mammal
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link