- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Gentiobiose Feeding in Gentian in vitro Overwintering Buds or Plantlets
Published: Vol 5, Iss 12, Jun 20, 2015 DOI: 10.21769/BioProtoc.1499 Views: 7942
Reviewed by: Tie LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1956 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1896 Views

CAPS-Based SNP Genotyping for Nitrogen-Response Phenotypes in Maize Hybrids
Jannis Jacobs [...] Peter K. Lundquist
Dec 20, 2025 550 Views
Abstract
To study the functions of sugars in plants, feeding experiment is one of the most common and easy methods. However, the traditional method, e.g., a floating of leaf discs on sugar-containing solution seems to have an insufficient efficiency of sugar uptake, despite of high osmotic and injury effects. This is a protocol to feed oligosaccharide gentiobiose into in vitro cultured tissues of gentian. This protocol enables to incorporate gentiobiose into intact tissues without exposure to osmotic stress and may be useful to other plant species that are able to propagate by shoot tip culture.
Keywords: GentianMaterials and Reagents
- Gentian (Gentiana triflora) tissue culture plantlets
- Sucrose
- Gentiobiose (Sigma-Aldrich, catalog number: G3000-5G )
- Gellan gum (Wako USA, catalog number: 075-03075 )
- Liquid nitrogen
- Milli Q grade water
- MS vitamins (see Recipes)
- Propagation medium (see Recipes)
- IOWB induction medium (see Recipes)
- Gentiobiose medium (see Recipes)
Equipment
- Sterile magenta boxes (sterilized by autoclaving)
- Surgical tape (3M, catalog number: 1530-0 )
- Glass culture tubes (sterilized by autoclaving)
- Silicon plugs (sterilized by autoclaving)
- Sterile 15 ml plastic tubes (such as Greiner Bio-One GmbH, catalog number: 188271 )
- Sterile syringe filter (Millipore, catalog number: SLGP033RS )
- Sterile petri dishes (IWAKI PUMPS, catalog number: SH90-20 )
- Cutoff filter (Millipore, catalog number: UFC5003BK )
- Growth chambers
- Clean bench
- Scalpel
- Forceps
- Ball miller
- Centrifuge
- Freeze dryer
Procedure
- Preparation and gentiobiose feeding of gentian IOWB and plantlets
- In vitro shoot cultures of gentian were prepared according to the method of Hosokawa et al. (1996).
- Transfer shoot tips (approximately 1 cm) to magenta boxes containing 70 ml of propagation medium and culture at 20 °C for 1 to 2 months under a 16/8 h light/dark photoperiod (Figure 1A).
- Transfer 3 to 5 shoot tips (approximately 1 cm) to a magenta box containing 70 ml of IOWB induction medium (Figure 1B) and culture at 20 °C for over 6 months under a 16/8 h light/dark photoperiod (Figure 1C).
- Harvest the produced IOWBs and cut into approximately 2 cm length pieces using a scalpel and forceps on a plastic petri dish (Figure 2A).
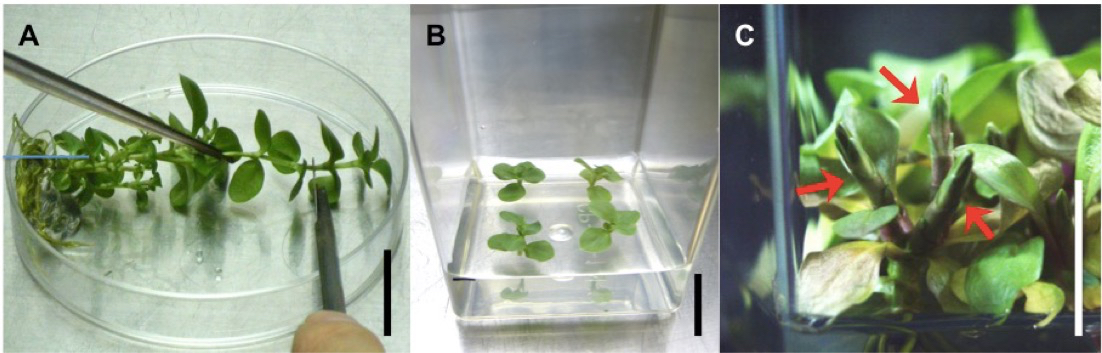
Figure 1. IOWBs induction from gentian plantlets. A. Cutting shoot tips from plantlet. B. Shoot tips placed on IOWB induction medium. C. IOWBs produced from gentian plantlets cultured for 6 months on IOWB induction medium. Arrows indicate IOWBs. Bar: 2 cm
- Transfer IOWBs or plantlets to glass culture tubes containing 2 ml of a gentiobiose medium and seal the top of the tubes with silicon plugs (Figure 2B).
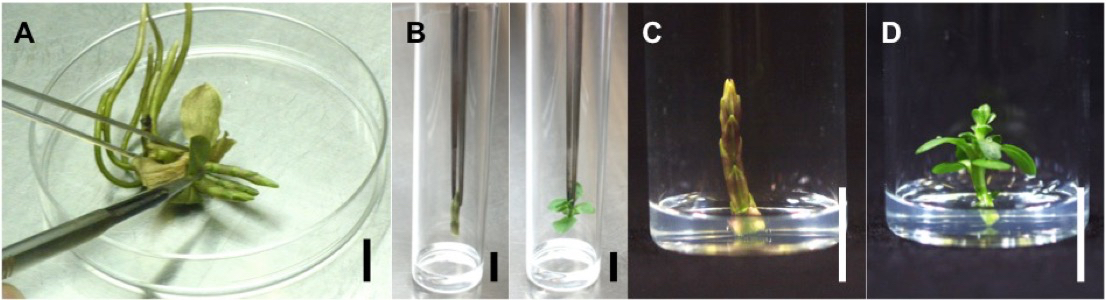
Figure 2. Gentiobiose feeding into gentian IOWB and plantlet. A. Harvest of produced IOWB. B. Placement of IOWB and shoot tip on gentiobiose medium. C. IOWB cultured on gentiobiose medium. D. Shoot tip cultured on gentiobiose medium. Bar: 1 cm
- Culture at 20 °C under a 16/8 h light/dark photoperiod for appropriate period (Figure 2 C-D).
- Harvest parts of the samples not in contact with the medium.
- In vitro shoot cultures of gentian were prepared according to the method of Hosokawa et al. (1996).
- Detection of gentiobiose in IOWBs or plantlets
- Freeze-dry and pulverize IOWBs or plantlets in a ball-miller.
- Homogenize 10 mg dry weight of samples with 500 μl of 50% (v/v) methanol.
- Centrifuge the homogenates at 20,000 x g for 5 min and filtrate the supernatants through a cutoff filter.
- Subject the filtrates to thin layer chromatography (Figure 3) or other analyses to detect gentiobiose.

Figure 3. Example analysis of gentiobiose incorporated in gentian plantlets. Extracts from leaves of plantlets cultured on normal MS medium (Control) or MS medium containing 0.05% gentiobiose labeled with rhodamine (Treated) for 3 days were subjected to TLC analysis. STD, 0.01% gentiobiose labeled with rhodamine.
- Freeze-dry and pulverize IOWBs or plantlets in a ball-miller.
Notes
- Propagation and feeding steps should be aseptically performed using a clean bench.
- Labeled gentiobiose should be used only for confirmation of incorporated gentiobiose.
- For details about gentian IOWBs and plantlets, see Imamura et al. (2014).
- For details about TLC analysis, see Takahashi et al. (2014).
- For gentiobiose labeling, see the following publications, Hase et al. (1978) and Fry (1997).
Recipes
- MS vitamins (100 ml)
10 g myo-inositol
50 mg nicotinic acid
50 mg pyridxine hydrochloride
10 mg thiamine hydrochloride
200 mg glycine
- Propagation medium (1L)
4.6 g MS salt
1 ml MS vitamins
30 g sucrose
2 g gellan gum
Adjust pH 5.7 with KOH and autoclave for 15 min
- IOWB induction medium (1L)
4.6 g MS salt
1 ml MS vitamins
60 g sucrose
2 g gellan gum
Adjust pH 5.7 with KOH and autoclave for 15 min
- Gentiobiose medium (1L)
4.6 g MS salt
1 ml MS vitamins
10 g gentiobiose or labeled gentiobiose*
2 g gellan gum
Adjust pH 5.7 with KOH and autoclave for 15 min
*Add to autoclaved MS medium after decrease in temperature to approximately 60 °C
*Labeled gentiobiose is used for confirmation of incorporated gentiobiose
Acknowledgments
This protocol is adapted from the following publications, Imamura et al. (2014) and Takahashi et al. (2014). This research was supported by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology.
References
- Fry, S. C. (1997). Novel ‘dot-blot’ assays for glycosyltransferases and glycosylhydrolases: optimization for xyloglucan endotransglycosylase (XET) activity. Plant J 11(5): 1141-1150.
- Hase, S., Ikenaka, T. and Matsushima, Y. (1978). Structure analyses of oligosaccharides by tagging of the reducing end sugars with a fluorescent compound. Biochem Biophys Res Commun 85(1): 257-263.
- Hosokawa, K., Nakano, M., Oikawa, Y. and Yamamura, S. (1996). Adventitious shoot regeneration from leaf, stem and root explants of commercial cultivars of Gentiana. Plant Cell Rep 15(8): 578-581.
- Imamura, T., Higuchi, A., Sekine, K. T., Yamashita, T. and Takahashi, H. (2014). High concentration of sucrose induces overwintering bud formation in gentian plantlets cultured in vitro. Plant Biotech 31(2): 97-104.
- Takahashi, H., Imamura, T., Konno, N., Takeda, T., Fujita, K., Konishi, T., Nishihara, M. and Uchimiya, H. (2014). The gentio-oligosaccharide gentiobiose functions in the modulation of bud dormancy in the herbaceous perennial gentiana. Plant Cell 26(10): 3949-3963.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Takahashi, H. and Nishihara, M. (2015). Gentiobiose Feeding in Gentian in vitro Overwintering Buds or Plantlets. Bio-protocol 5(12): e1499. DOI: 10.21769/BioProtoc.1499.
Category
Plant Science > Plant physiology > Plant growth
Plant Science > Plant physiology > Nutrition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









