- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Simple Digital Photography for Assessing Biomass and Leaf Area Index in Cereals
Published: Vol 5, Iss 11, Jun 5, 2015 DOI: 10.21769/BioProtoc.1488 Views: 12362
Reviewed by: Samik BhattacharyaMaria SinetovaJoern Klinkenberg

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1960 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1898 Views

ClearDepth Method for Evaluations of Root Depth in Soil-Filled Pots
Michel Ruiz Rosquete [...] Wolfgang Busch
Aug 20, 2025 2131 Views
Abstract
These instructions refer to obtaining fast and low-labour estimates of ground cover, leaf area index and green biomass for a large number of plots, as those encountered in cereal breeding programs. The procedure includes obtaining the pictures in the field and processing them once downloaded to a computer.
Keywords: Image processingMaterials and Reagents
- Crop
Note: The crop must be free of green weeds. The presence of spikes, flowers, etc. on the external part of the canopy may require using a specific calibration curve for that development stage.
Equipment
- Conventional digital camera.
Note: This is the only equipment required once the method has been calibrated for a range of crops similar to those where it has to be applied. Any commercial camera, compact or reflex, is suitable.
- A digital area meter is required for calibrating leaf area index (LAI, e.g. DIAS II, Delta-T Devices)
- A drying oven is required for calibrating biomass (e.g. Memmert, model: 800 , minimum precision required: 2 °C)
- A precision balance is required for calibrating biomass (e.g. Mettler Toledo, model: PB3002-L , minimum precision required: 0.1 g)
Software
- Image processing software that allows quantifying the average colour of the whole picture
Note: The program BreedPix 2.0 described in Casadesús and Villegas (2014) is a suitable tool for processing a large number of pictures with the minimal required steps by the user. It can be obtained for free from the authors. General-purpose free software for quantitative image analysis (such as ImageJ or Fiji) can also be used but in this case a macro should be written specifically for this protocol.
Procedure
The pictures must be obtained under stable lighting conditions for the whole sampling session. This can be either under clear sky or under moderately cloudy conditions if these are equivalent for all the plots. Also, to avoid differences in the length of shadows in the pictures the time of day would be preferably ± 3 h around solar noon. Wind conditions should be avoided because bent canopies may expose to the camera a different amount of their leaf area and different parts of the plants than in normal conditions.
- Acquiring the images in the field
- Predefine the structure and order of the series of photographs.
In order to facilitate the identification of each image, the plots of the field trial must be photographed following a predefined plan (Figure 1). The plan must also specify the number of photographs to take at each plot, a decision that would depend on the area/ size of the plots and their internal heterogeneity. For instance, for plots under 2 m2 only one image is feasible to obtain, while for a plot area in the range of 20 m2, the number of photographs may be between 3 and 5 depending on the internal heterogeneity of the plots. If a plot is highly regular, a lower number of pictures may be representative. On the other hand, plots with different plant densities will need more pictures in order to account for all the plants contained in it. It is advised to include regularly in the series of photographs some additional pictures to help confirm the identification of the neighbouring images. For instance, pictures of some label, the perspective of the field trial from the current position, etc. will be useful for verifying the position of each photograph within the field when they will be downloaded for analysis.
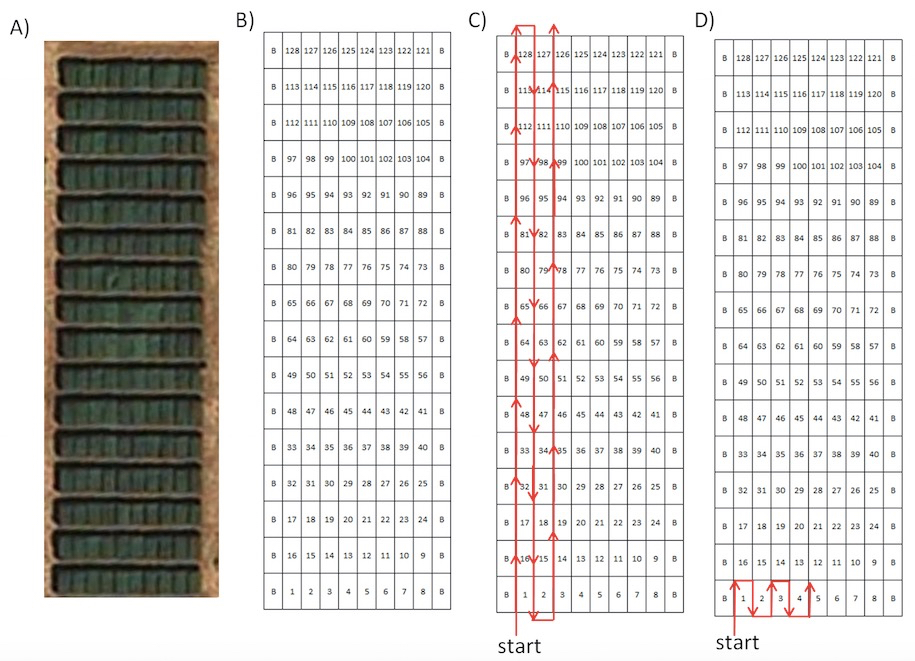
Figure 1. A) Orthoimage of a typical experimental or breeding field experiment with several plots B) Field experimental map C) Possibility 1 of pre-defined path D) Possibility 2 of pre-defined path. Within the maps, “B” indicates “border plot”.
- Camera settings.
The camera settings can either be left to the default options or optimized according to the photographer’s criteria but must be the same for all pictures. Under stable lighting conditions the settings can be set to automated mode. The focal length is not expected to have a remarkable effect on these assessments.
The zoom should be fixed -the same for all pictures- to one position which allows a wide view of the plot without including any part external to the sampled plot - e.g. neighbour plots, spacing between plots, photographer’s feet, etc.
The images can be saved in JPG format, at an image size equal or larger than 1024 x 768 pixels. The only problem with large images is that they require more memory in the camera and will take longer to process in the computer.
- Taking the pictures.
The photographer will follow the plots according to the sequence specified in the sampling plan. The camera must be held oriented zenithally - that is, aiming exactly downwards. The pictures can be obtained holding the camera with one hand, with the photographer’s arm fully extended ahead, above the canopy, at shoulder height. Care must be taken to not include the photographer’s feet in the image. Also, the gap between two plots should not appear in the picture.
- Predefine the structure and order of the series of photographs.
- Processing the images
Once the images are downloaded to the computer, image-processing software must be used for calculating one or more vegetation indices for each of the images. The easiest index to quantify and to interpret is the Green Fraction (GF), which in early stages of the crop is closely related with ground cover and it can be calculated as the proportion of green pixels to the total number of pixels of the image.
GF = green pixels/total pixels
The recommended scope for using this method in winter cereals is for early stages above anthesis. At later stages the spikes have an effect on the properties of the canopies and, also, vegetation indices use to become saturated when LAI is above 3 (Aparicio et al., 2000).
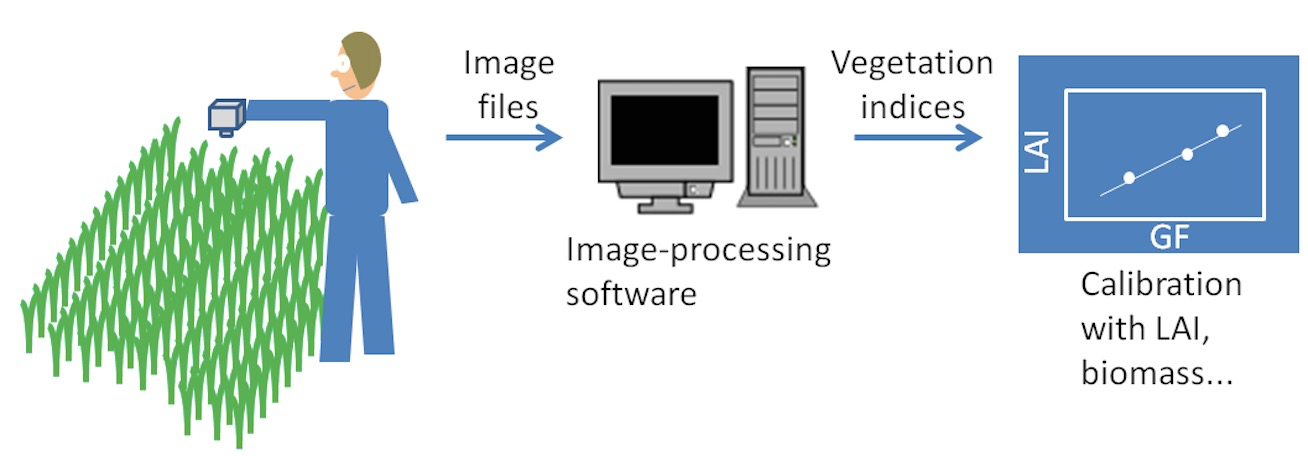
Figure 2. Process of obtaining the photographic sampling at the field, processing the images to calculate some vegetation indices and converting those indices to indirect assessments of LAI or green biomass
The classification of the pixels as green can be based on its Hue. When pixel colours are expressed in the HIS colour coordinates (for Hue, Intensity, and Saturation), green pixels are those whose Hue value ranges between 60º and 180º (approximately between yellow-lime and turquoise). Other vegetation indices based on photographs are exposed in Casadesus et al. (2007) and Casadesus and Villegas (2014). Briefly, once each of the pixels in an image has been classified according to its Hue as either green vegetation or background, GF can be quantified as the ratio of green vegetation pixels to total pixels.
The program BreedPix 2.0 (Casadesus and Villegas, 2014) can calculate the GF of hundreds of images within seconds after downloading the camera to a computer. In this software, the user just has to select the folder that contains the image files to process. Then it will calculate the GF corresponding to each photograph and it will write the results in an output file for the whole list of photographs. Optionally, the user can indicate the path followed to obtain the pictures, which will facilitate identifying the genotype at each plot. As an alternative to BreedPix, most tools for quantitative image analyses (e.g. ImageJ, Fiji, etc.) include methods for counting how many pixels lay on a given range of colours and this feature can be used to quantify green pixels on individual images or portions of the image.
- Indirect assessment of LAI and Green Biomass
The image-processing software will calculate a vegetation index for each picture which is strongly correlated with both LAI and Green Biomass. In order to obtain an estimate of the LAI or the Green Biomass of that plot it is necessary to have a calibration curve for a similar plant material where to interpolate the value for the vegetation index. For instance, all winter cereals (wheat, barley, oats, etc.) could share a common calibration curve before anthesis. Instead, an alfalfa crop should have a different calibration curve due to a different leaf distribution within the canopy. To construct a calibration curve for a particular plant material it is necessary to harvest the samples of that crop (immediately after the photograph) with different plant densities or at different developing stages, for the destructive lab-determination of LAI and/or green biomass. Determination of LAI requires measuring with a leaf-area meter the area of the green leaves (one side laminae). Determination of green biomass in terms of crop dry weight (CDW) requires weighing jointly the sampled green parts - leaves, stems and spikes if present– after oven-drying them at 70 °C for 48 h. In all cases, a representative sample of the plot must be measured, of at least all the plants contained in a 0.075 m2 section of the plot. A representative sample may be chosen at random after discarding two zones: the 10% of the plot with higher biomass and the 10% of the plot with lower biomass, both estimated visually.
For instance, in winter cereal crops the calibration curve should contain points from seedling (Zadoks stage 12) to anthesis (Zadoks stage 65). The plant densities must cover the expected range where the calibration will be used.
An example of calibration curve for winter cereals at pre-anthesis is shown in Figure 5, based on Casadesus and Villegas (2014). From that example, the calculation for converting GF to LAI is:
LAI = 3.164 x GF - 0.143
Based on the same source, the calculation of CDW is:
CDW = 440.2 x GF - 56.81
Representative data
- Figure 3 is an example of how the pictures should look like. Figure 4 shows a sample of several pictures obtained in a sampling session. Figure 5 shows an example of calibration curve for LAI.

Figure 3. Example of one zenithal photograph valid for assessing green biomass

Figure 4. Example of the sequence of pictures obtained in a sampling session. In this case, there were 6 images per plot, with an additional image between plots- showing a perspective of the field trial- to help identify the neighbouring images. This figure shows only part of the sample; the whole session may include some hundreds of images.
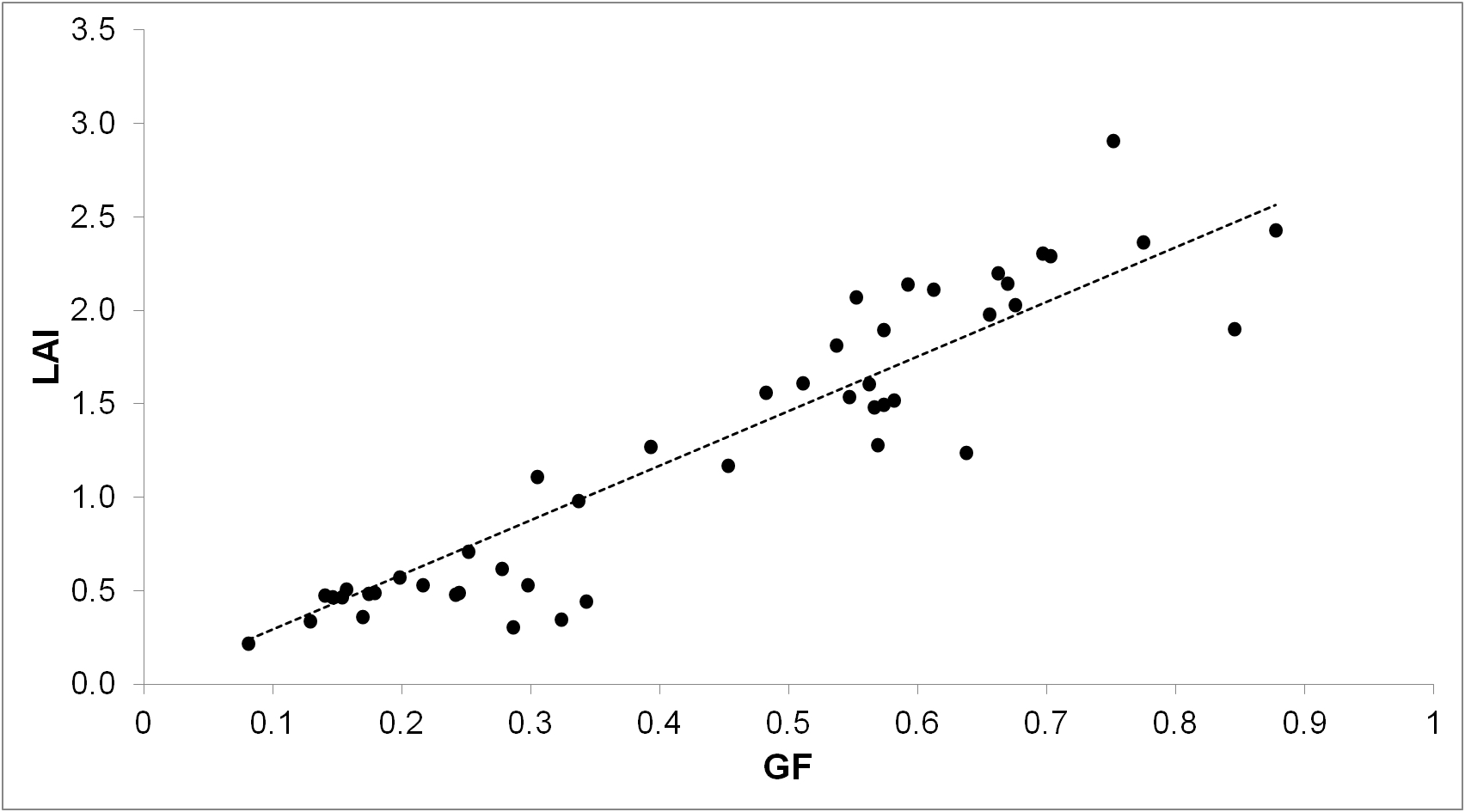
Figure 5. Example of a general calibration curve for LAI in winter cereals. GF was calculated from photographs obtained just before sampling destructively for LAI in 24 plots including bread wheat, triticale and tritordeum, at two different dates before anthesis.
Acknowledgments
This protocol was adapted from Casadesus et al. (2007) and Casadesús and Villegas (2014).
References
- Aparicio, N., Villegas, D., Casadesús, J., Araus, J. L. and Royo, C. (2000). Spectral vegetation indices as non-destructive tools for determining durum wheat yield. Agron J 92: 83-91.
- Casadesús, J. and Villegas, D. (2014). Conventional digital cameras as a tool for assessing leaf area index and biomass for cereal breeding. J Integr Plant Biol 56(1): 7-14.
- Casadesus, J., Kaya, Y., Bort, J., Nachit, M. M., Araus, J. L., Amor, S., Ferrazzano, G., Maalouf, F., Maccaferri, M., Martos, V., Ouabbou, H. and Villegas, D. (2007). Using vegetation indices derived from conventional digital cameras as selection criteria for wheat breeding in water-limited environments. Ann Appl Biol 150, 227-236.
- Zadoks, J. C., Chang, T. T. and Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed Res 14, 415-421.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Casadesús, J. and Villegas, D. (2015). Simple Digital Photography for Assessing Biomass and Leaf Area Index in Cereals. Bio-protocol 5(11): e1488. DOI: 10.21769/BioProtoc.1488.
Category
Plant Science > Plant physiology > Plant growth
Plant Science > Plant physiology > Phenotyping
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









