- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Establishment of a Symbiotic in vitro System between a Green Meadow Orchid and a Rhizoctonia-like Fungus
Published: Vol 5, Iss 10, May 20, 2015 DOI: 10.21769/BioProtoc.1482 Views: 11834
Reviewed by: Arsalan DaudiKanika GeraAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Simplifying Barley Leaf Rust Research: An Easy and Reproducible Infection Protocol for Puccinia hordei on a Small Laboratory Scale
Caroline I. Skoppek and Jana Streubel
Jul 20, 2023 1706 Views

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1959 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1897 Views
Abstract
Symbiotic orchid seed germination in an in vitro system allows the growth of mycorrhizal protocorms and plantlets for scientific purposes. Orchids in nature need to establish a mycorrhizal symbiosis with fungal partners to germinate and develop into adult plants. Here we present a protocol for symbiotic germination of the terrestrial Mediterranean green meadow orchid Serapias vomeracea. The fungal symbiont Tulasnella calospora (T. calospora) (Basidiomycetes, Cantharellales) was chosen because of its common occurrence (Girlanda et al., 2011), its ability to grow in culture and compatibility in germination assays. T. calospora is one of the most common rhizoctonia-like fungi associated with terrestrial as well as epiphytic orchids.
Materials and Reagents
- Serapias vomeracea seeds (collected in the field, in the north-west Mediterranean meadows of Italy; seeds from this orchid species could be retrieved through “index seminum” of international botanic gardens or germplasm banks)
- Tulasnella calospora inoculum (strain MUT 4182) (previously grown in sterile culture on MEA medium)
- Sterile double deionized water (neutral pH)
- Tween-20 (Sigma-Aldrich, catalog number: P2287 )
- Milled oats (retrieved from organic shops)
- Malt Extract (Sigma-Aldrich, Fluka, catalog number: 70167 )
- Ethanol (Sigma-Aldrich, Fluka, catalog number: 0 2860 )
- 5% sodium hypochlorite (commercial bleach)
- Agar (Sigma-Aldrich, catalog number: A1296 )
- Glucose (Sigma-Aldrich, catalog number: G8270 )
- Peptone (Sigma-Aldrich, Fluka, catalog number: P5905 )
- Sterilizing solution (see Recipes)
- Solid oat medium (see Recipes)
- MEA medium (see Recipes)
Equipment
- Balance
- Small spatula
- Small metal forceps
- Sterile 10 x 10 mm Whatman No.1 filter paper
- Lancets
- 2.0 ml centrifuge tubes
- Micropipette
- Filter tips
- Centrifuge
- Vortex
- Timer
- Laminar flow hood
- Petri dishes (9 cm diameter)
- Parafilm (Sigma-Aldrich, catalog number: P7793 )
- Bunsen burner
- Climatic chamber (Binder GmbH)
Procedure
- Wipe the working area with ethanol and alcohol/flame sterilize the spatula.
- Wipe the laminar flow hood and alcohol/flame sterilize the forceps and the lancets. All steps must be carried out under laminar flow hood.
- Fill about 25 mg of Serapias vomeracea seeds for each 2.0 ml centrifuge tube, being careful to bring down all the seeds to the bottom of the centrifuge tube. For this purpose, centrifuge all the tubes at maximum speed with their lids open for 1 min. Make sure not to leave any remains of seeds on the cap edge; these seeds will not be sterilized and may contaminate those sterilized.
- Sterilize seeds adding 1 ml of sterilizing solution for each tube and start immediately the timer; mix well and place on vortex for 18 min.
- After this, centrifuge at maximum speed for 1 min and, quickly replace Sterilizing solution with 1 ml of sterile deionized water. Incubate for 5 min and repeat this washing step three times. To avoid collecting seeds in the tip while change washing water, briefly centrifuge to bring down all the seeds to the bottom of the centrifuge tube.
Note: Steps 4 and 5 must be completed within 20 min.
- Using sterile forceps, spread 4-10 squares of Whatman No.1 filter paper (10 x 10 mm) spaced equally on a petri dish filled with 25 ml of Solid oat medium as shown in the Figure 1. Gently place the seeds spread out on the filter paper squares using pipette and filter tips. Normally it is recommended to prepare from 10 to 20 petri dishes.
Note: each filter piece of paper will be a replicate and the number of squares indicated above is the minimum and the maximum to use in a single petri dish, as how many replicates you need.
- Inoculate each petri dish with Tulasnella calospora (AL13/D strain). Inoculum consist of a 3 x 3 mm portion of actively growing mycelium (from pure culture on MEA medium) cut with sterile lancets from the petri dish of the fungal pure culture, placed in the center of capsule using sterile lancets (Figure 1). Control plates were left uninoculated.
- Seal petri dishes with Parafilm and aluminum sheet (to ensure darkness) and place in a climatic chamber at stable temperature of 20 °C (Figure 2).
- Seeds are considerable to be germinated when protocorms developed from imbibed seeds after 15-30 days. After this time, well developed protocorms, start to grow in plantlets with leaves. Well-developed plantlets may be exposed at light with photoperiod of 16 h of light (20-25 °C) and 8 h of dark (18-20 °C) (Figure 3).
Representative data
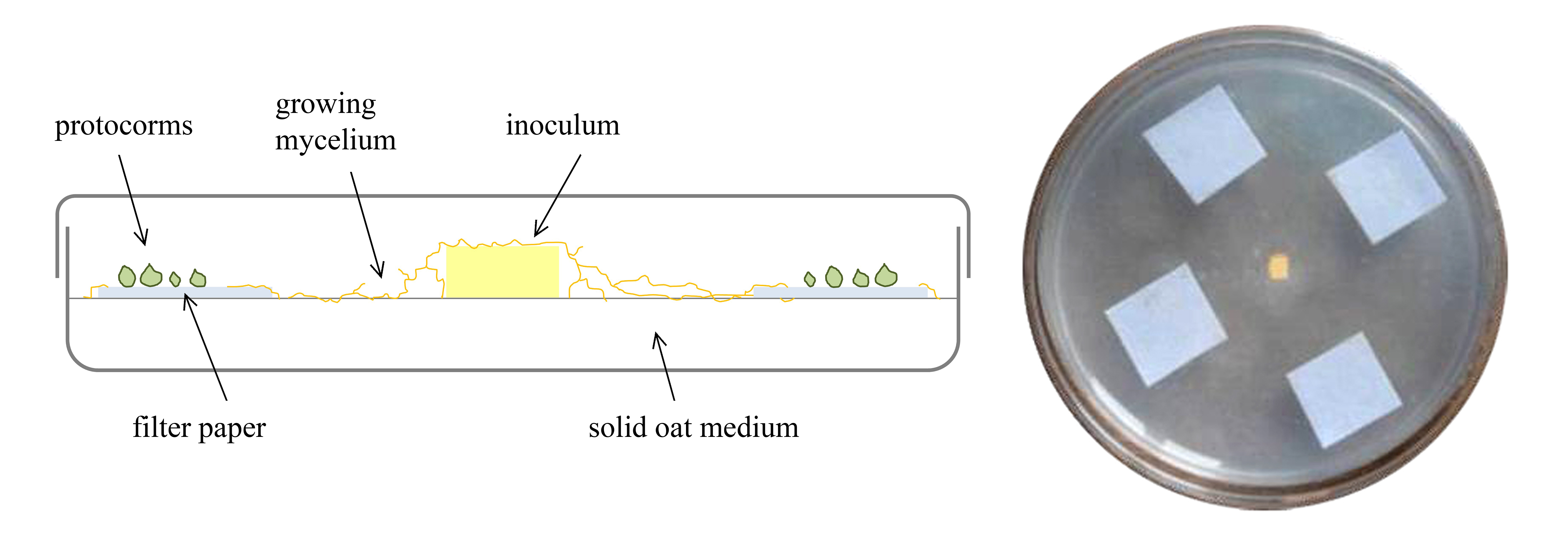
Figure 1. Representative scheme of germination assay [modified from Ercole (2014)]

Figure 2. Developed protocorms after 15-30 days of dark incubation

Figure 3. Developed protocorms after 45 days (30 days of darkness and 15 days of light exposure)
The reproducibility of the symbiotic seed germination of S. vomeracea with T. calospora is usually successful. In some cases we noted low germination percentage due to seed viability mainly. Viability test on seeds allow to evaluating a priori the success rate. Percentage of germination can be evaluated by count of all seeds at time zero (all seeds sown) and the germinated seeds at time 15, 30 and 45 days. Usually we reported high percentage of germination (from 95% at time 15 days, to 60% at time 45 days). The decrease of the percentage of germination from time 15 days to time 45 days is mainly due to “natural selection” and/or competition between seeds (seeds with a high growth rate grow at the detriment of slower).
Notes
Note that this protocol can be applied to many different orchid and fungus species, with some modification in the sterilization time of seeds. Every orchid species has different seeds with different teguments, so the sterilization time depends on this. In some cases success of germination depends on compatibility between orchid and fungus; indeed, not all fungi (also in the Rhizoctonia-form genus) can germinate orchid seeds.
Recipes
- Sterilizing solution (1% sodium hypochlorite) (100 ml)
Sodium hypochlorite 5% (w/v) 20 ml
Add deionized water to 100 ml final volume
Stir well to mix
Add 100 µl of Tween-20 to the solution
- Solid oat medium (0.3% milled oats, 1% agar) (1 L)
Milled oats 3 g
Agar 10 g
Add deionized water to 1 L final volume
Autoclave for 20 min
- MEA medium (1.8% malt extract, 2.0% agar, 2.0% glucose, 0.2% peptone) (1 L)
Malt Extract 18 g
Agar 20 g
Glucose 20 g
Peptone 2 g
Add deionized water to 1 L final volume
Autoclave for 20 min
Acknowledgments
This protocol was adapted from the following publications: Perotto et al. (2014); Ercole et al. (2013); Ercole (2014).
Research on orchid mycorrhiza was partly funded by the Italian MIUR and by local funding from the University of Torino.
References
- Ercole, E. (2014). Micorrize e conservazione delle orchidee. In: Adamo, M., Masella, E. A., Bonini, I., Borzatti, A., Buono, S., Carasso, V., Castagnini, P., Ceriani, R. M., Chioccia, G., Ciaschetti, G., Vitis, M. D., Martino, D. L., Ercole, E., Fabrini, G., Fay, M. F., Fonck, M., Gallino, B., Gransinigh, E., Haile, G., Legitimo, I., Maccherini, S., Magrini, S., Mannocci, M., Marks, T. R., Mazzoncini, V., Pellegrino, G., Pierce, S. and Pirondini, A. (eds). Esperienze di conservazione delle orchidee. Viterbo: Tipoligrafia Quatrini, vol. 1, p. 17-23.
- Ercole, E., Rodda, M., Molinatti, M., Voyron, S., Perotto, S. and Girlanda, M. (2013). Cryopreservation of orchid mycorrhizal fungi: a tool for the conservation of endangered species. J Microbiol Methods 93(2): 134-137.
- Girlanda, M., Segreto, R., Cafasso, D., Liebel, H. T., Rodda, M., Ercole, E., Cozzolino, S., Gebauer, G. and Perotto, S. (2011). Photosynthetic Mediterranean meadow orchids feature partial mycoheterotrophy and specific mycorrhizal associations. Am J Bot 98(7): 1148-1163.
- Perotto, S., Rodda, M., Benetti, A., Sillo, F., Ercole, E., Rodda, M., Girlanda, M., Murat, C. and Balestrini, R. (2014). Gene expression in mycorrhizal orchid protocorms suggests a friendly plant-fungus relationship. Planta 239(6): 1337-1349.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ercole, E., Rodda, M., Girlanda, M. and Perotto, S. (2015). Establishment of a Symbiotic in vitro System between a Green Meadow Orchid and a Rhizoctonia-like Fungus. Bio-protocol 5(10): e1482. DOI: 10.21769/BioProtoc.1482.
Category
Plant Science > Plant physiology > Endosymbiosis
Plant Science > Plant physiology > Plant growth
Microbiology > Microbe-host interactions > Fungus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









