- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
RNA-binding Protein Immunoprecipitation (RIP) to Examine AUF1 Binding to Senescence-Associated Secretory Phenotype (SASP) Factor mRNA
Published: Vol 5, Iss 10, May 20, 2015 DOI: 10.21769/BioProtoc.1481 Views: 15262
Reviewed by: HongLok LungJyotiska ChaudhuriAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Individual-nucleotide-resolution UV Cross-linking and Immunoprecipitation (iCLIP) of UPF1
David Zünd and Oliver Mühlemann
Apr 5, 2014 15087 Views

Separation and Visualization of Low Abundant Ubiquitylated Forms
Ramona Schuster [...] Mafalda Escobar-Henriques
Nov 20, 2018 5520 Views

Assessing Self-interaction of Mammalian Nuclear Proteins by Co-immunoprecipitation
Claudia Cattoglio [...] Anders S. Hansen
Feb 20, 2020 9474 Views
Abstract
Immunoprecipitation and subsequent isolation of nucleic acids allows for the investigation of protein:nucleic acid interactions. RNA-binding protein immunoprecipitation (RIP) is used for the analysis of protein interactions with mRNA. Combining RIP with quantitative real-time PCR (qRT-PCR) further enhances the RIP technique by allowing for the quantitative assessment of RNA-binding protein interactions with their target mRNAs, and how these interactions change in different cellular settings. Here, we describe the immunoprecipitation of the RNA-binding protein AUF1 with several different factors associated with the senescence-associated secretory phenotype (SASP) (Alspach and Stewart, 2013), specifically IL6 and IL8. This protocol was originally published in Alspach et al. (2014).
Materials and Reagents
- Tissue culture
- DMEM (Sigma-Aldrich, catalog number: D6429 )
- Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F2442 )
- Medium M199 (Sigma-Aldrich, catalog number: M7528 )
- Penicillin/streptomycin (Sigma-Aldrich, catalog number: P4333 )
- BJ human foreskin fibroblasts
- Bleomycin sulfate (Sigma-Aldrich, catalog number: B8416 )
- BJ culture media (see Recipes)
- DMEM (Sigma-Aldrich, catalog number: D6429 )
- General materials
- Bradford Protein Assay reagent (Bio-Rad Laboratories, catalog number: 500-0006 )
- Polyclonal anti-AUF1 antibody (EMD Millipore, catalog number: 07260MI )
- Normal Rabbit IgG antibody (Cell Signaling Technology, catalog number: 2729 )
- Protein A Dynabeads (Life Technologies, catalog number: 10002D )
- MCLB (mammalian cell lysis buffer) (see Recipes)
- NT2 buffer
(see Recipes)
- Buffer A (see Recipes)
- Buffer B (see Recipes)
- Buffer C (see Recipes)
- Bradford Protein Assay reagent (Bio-Rad Laboratories, catalog number: 500-0006 )
- Immunoprecipitation reagents
- Tris
- EDTA
- NP40
- NaCl
- Phosphate-buffered saline (PBS)
- SDS
- Deoxycholate
- MgCl2
- NaH2PO4
- RNAse OUT (Life Technologies, catalog number: 10777-019 )
- Proteinase K (Sigma-Aldrich, catalog number: P6556 )
- RiboPure RNA isolation kit (Life Technologies, catalog number: AM1924 )
- Tris
- qRT-PCR reagents
- dNTPs
- Hexanucleotide mix (Roche Diagnostics, catalog number: 11277081001 )
- SuperScript II Reverse Transcriptase (Life Technologies, catalog number: 18064-014 )
- Power SYBR GREEN PCR master mix (Life Technologies, catalog number: 4367659 )
- dNTPs
- Primers
- IL6 forward: 5′-ACA TCCTCGACGGCA TCTCA-3′
- IL6 reverse: 5′-TCACCAGGCAAGTCTCCTCA-3′
- IL8 forward: 5′-GCTCTGTGTGAAGGTGCAGT-3′
- IL8 reverse: 5′-TGCACCCAGTTTTCCTTGGG-3′
- IL6 forward: 5′-ACA TCCTCGACGGCA TCTCA-3′
Equipment
- Tissue Culture
- 37 °C forced-air incubator maintained at 5% CO2 and 5% O2 (Thermo Fisher Scientific, Forma Series II water jacketed CO2 incubator)
- Laminar-flow biosafety cabinet (Labconco Purifier Class II Biosafety Cabinet)
- 37 °C forced-air incubator maintained at 5% CO2 and 5% O2 (Thermo Fisher Scientific, Forma Series II water jacketed CO2 incubator)
- Immunoprecipitation
- Spectrophotometer capable of reading at 450 and 595 nm
- Dyna-mag magnetic bead separator (Life Technologies, catalog number: 12321D )
- Micro centrifuge
- End-over-end tube rotator (Thermo Fisher Scientific, catalog number: 400110Q )
- Spectrophotometer capable of reading at 450 and 595 nm
- qRT-PCR
- PCR machine (Bio-Rad Laboratories, model: C1000 thermo cycler )
- Real-time PCR detection system (Bio-Rad Laboratories, model: CFX96 Real Time System )
- PCR machine (Bio-Rad Laboratories, model: C1000 thermo cycler )
- General equipment
- Tissue culture cell scrapers
- Refrigerated micro centrifuge
- Heat block capable of reaching 70 °C
- Tissue culture cell scrapers
Procedure
- BJ human foreskin fibroblasts were cultured at 37 °C, 5% CO2, 5% O2 in BJ culture media. Stress-induced premature senescence (SIPS) was induced by treating the cells with 100 μg/ml bleomycin sulfate for 24 h. Bleomycin sulfate-containing media was replaced with fresh media following the 24 h treatment. Cells are senescent 3 days following treatment with bleomycin.
- Collect cells by scraping in PBS on ice (for a 15 cm culture dish, 4 ml of PBS was used). 3.5 million cells are required for each immunoprecipitation (IP) (IgG control and specific antibody). Cell number was determined using a hemocytometer.
- Pellet cells by centrifugation at 1,500 rpm for 5 min at 4 °C.
- To lyse cells, resuspend the pellet in 1 ml MCLB and
incubate for 20 min at 4 °C with end-over-end rotation.
- Centrifuge cell lysates at maximum speed for 20 min at 4 °C to remove
cellular debris. Remove 1 ml of cell lysate to a micro centrifuge tube.
- Remove 100 μl of protein lysate from each and place in a new Eppendorf tube. This will be used as the input for each sample.
- Quantification of protein concentration was performed using the Bradford assay following manufacturer’s protocols.
- Prepare the magnetic beads for immunoprecipitation.
- Wash 100 μl beads per IP three times in 0.1 M NaH2PO4 (for
example: If 2 AUF1 immunoprecipitations and 2 IgG immunoprecipitations are to be performed, you will require 200 μl of beads for the AUF1 IPs and 200 μl of beads for the IgG IPs). Washes are performed by resuspending the magnetic beads, and then separating them from solution using a magnetic micro centrifuge tube rack.
- Resuspend washed beads in 500 μl 0.1 M NaH2PO4 and add 30 μg anti-AUF1 or IgG control antibody per IP (for example: If you are performing 2 AUF1 IPs, resuspend your beads in 500 μl of 0.1 M NaH2PO4 and add 60 μg of anti-AUF1 antibody).
- Incubate beads with antibodies at room temperature for at least 1 h with end-over-end rotation.
- Wash antibody-conjugated beads 3 times in buffer A by resuspending beads in buffer A and pulling them down using a magnetic micro centrifuge tube rack. Leave beads in buffer A until ready for IP.
- Wash 100 μl beads per IP three times in 0.1 M NaH2PO4 (for
example: If 2 AUF1 immunoprecipitations and 2 IgG immunoprecipitations are to be performed, you will require 200 μl of beads for the AUF1 IPs and 200 μl of beads for the IgG IPs). Washes are performed by resuspending the magnetic beads, and then separating them from solution using a magnetic micro centrifuge tube rack.
- Pre-clear 3 mg of protein per IP by incubating with 20 μl non-antibody conjugated beads. Bring up to 1 ml volume with MCLB. Incubate for 30 min at 4 °C with end-over-end rotation.
- At the same time, resuspend antibody-conjugated beads in NT2 buffer. At this time you need to aliquot the beads for the individual IPs. For example, if you are preparing to do 2 AUF1 IPs, resuspend the beads in 200 μl NT2 buffer and separate them in to 2 tubes of 100 μl each. Incubate for 30 min at room temperature.
- Immunoprecipitate overnight:
- Remove the beads from the pre-cleared protein lysate using a
magnetic micro centrifuge tube rack, keeping the protein lysate and discarding the beads.
- Remove the antibody-conjugated beads from NT2 buffer using a
magnetic micro centrifuge tube rack, keeping the beads and discarding the NT2 buffer.
- Resuspend antibody-conjugated beads in pre-cleared protein
lysate.
- Place tubes on end-over-end rotator. Incubate at 4 °C overnight.
- Remove the beads from the pre-cleared protein lysate using a
magnetic micro centrifuge tube rack, keeping the protein lysate and discarding the beads.
- Wash the beads twice in each of the following buffers by resuspending the beads and then separating them from solution using a magnetic micro centrifuge tube rack
- Buffer A
- Buffer B
- Buffer C
- Buffer A
- Incubate beads for 30 min at 55 °C in 100 μl NT2 buffer supplemented with:
- 0.1% SDS
- 80 U RNAse OUT
- 30 μg proteinase K
- 0.1% SDS
- Extract the RNA from the IP’s and input samples by adding 1 ml of Trizol (included in the RiboPure RNA isolation kit) to the mixture described in step 12. Incubate the solution for 5 min at room temperature. Proceed with the RNA isolation protocol provided in the RiboPure RNA isolation kit. Elute the RNA in 24 μl H2O.
- The eluted RNA can now be used to generate cDNA for qRT-PCR.
- cDNA was synthesized using hexanucleotide primers and
following the manufacturer’s protocol provided with Super Script II
Reverse Transcriptase.
- PCR was performed with Power SYBR Green master mix following the manufacturer’s protocol. The annealing temperature used for the primers that are outlined in the Materials and Reagents section is 60 °C.
- cDNA was synthesized using hexanucleotide primers and
following the manufacturer’s protocol provided with Super Script II
Reverse Transcriptase.
Representative data
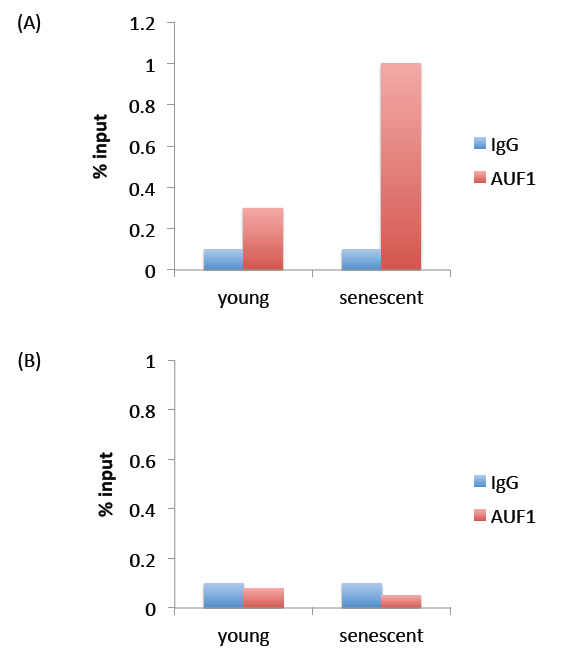
Figure 1. Representative results. Panel (A) represents expected results. The % input observed for AUF1 immunoprecipitation is above that observed for IgG immunoprecipitation, indicating that the AUF1 antibody pulled down more protein (and RNA) than the non-specific IgG antibody. Panel (B) represents a negative result, where immunoprecipitation with the AUF1 antibody did not pull down more protein (and RNA) than the non-specific IgG antibody.
Notes
- Please see Figure 2 for a simplified experimental workflow.
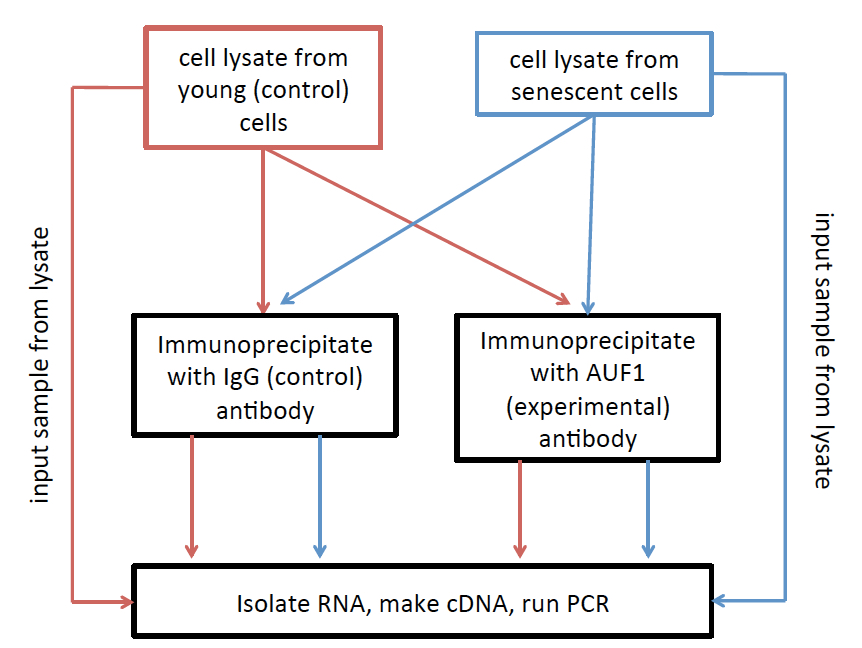
Figure 2. RNA Immunoprecipitation (RIP) workflow. Lysates from each treatment are divided into three groups: 1) Inputs, which are used to normalize to overall expression level of the RNAs of interest, 2) IgG immunoprecipita8ons, which are used to set the background level of immunoprecipitation, and 3) AUF1 immunoprecipita8ons, used to investigate the relative level of AUF1 binding to your RNA of interest.
Recipes
- BJ culture media
DMEM
15% heat-inactivated fetal bovine serum
15% medium 199
1% penicillin/streptomycin
- MCLB (mammalian cell lysis buffer)
50 mM Tris (pH 8.0)
5 mM EDTA
0.5% NP-40
100 mM NaCl
- NT2 buffer
50 mM Tris (pH 7.4)
150 mM NaCl
1 mM MgCl2
- Buffer A
1x PBS
0.1% SDS
0.3% deoxycholate
0.3% NP-40
- Buffer B
5x PBS
0.1% SDS
0.5% deoxycholate
0.5% NP-40
- Buffer C
50 mM Tris-HCl (pH 7.4)
10 mM MgCl2
0.5% NP-40
Acknowledgments
This work was supported by NIH 5 R01 CA130919 (SAS), NIH Cellular Biochemical and Molecular Sciences Predoctoral Training Grant T32 GM007067 (E A), and an American Cancer Society Research Scholar Award (to S. A. Stewart). We thank Dr. Nicholas Davidson (Washington University School of Medicine) for his technical support in the generation of this protocol.
References
- Alspach, E., Fu, Y. and Stewart, S. A. (2013). Senescence and the pro-tumorigenic stroma. Crit Rev Oncog 18(6): 549-558.
- Alspach, E., Flanagan, K. C., Luo, X., Ruhland, M. K., Huang, H., Pazolli, E., Donlin, M. J., Marsh, T., Piwnica-Worms, D., Monahan, J., Novack, D. V., McAllister, S. S. and Stewart, S. A. (2014). p38MAPK plays a crucial role in stromal-mediated tumorigenesis. Cancer Discov 4(6): 716-729.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Alspach, E. and Stewart, S. A. (2015). RNA-binding Protein Immunoprecipitation (RIP) to Examine AUF1 Binding to Senescence-Associated Secretory Phenotype (SASP) Factor mRNA. Bio-protocol 5(10): e1481. DOI: 10.21769/BioProtoc.1481.
Category
Molecular Biology > RNA > RNA-protein interaction
Molecular Biology > RNA > qRT-PCR
Biochemistry > Protein > Immunodetection > Immunoprecipitation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









