- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Transient Transformation of Artemisia annua
Published: Vol 5, Iss 10, May 20, 2015 DOI: 10.21769/BioProtoc.1476 Views: 8728
Reviewed by: Tie LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1956 Views

A Novel Gene Stacking Method in Plant Transformation Utilizing Split Selectable Markers
Guoliang Yuan [...] Xiaohan Yang
Feb 20, 2025 1979 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1896 Views
Abstract
Transient transformation of Artemisia annua does not depend on chromosomal integration of heterologous DNA, and recombinant DNA can be introduced into plant cells via Agrobacterium aided by vacuum. The leaves of 7th and 8th internode from 4-week-old seedlings were chosen as explants, a vacuum system was applied to facilitate agrobacteria into plant cells, the co-cultivation was in the dark at 25 °C for 36-72 h, then GUS or GFP maker genes were used for testing the efficiency of the transformation. The method is used for quick transferring of genes into Artemisia annua (A. annua) by transient transformation.
Keywords: Artemisia annuaMaterials and Reagents
- Artemisia annua leaves from 4-week-old aseptic seedlings
- Agrobacterium tumefaciens (A. tumefaciens) strain EHA105 (or LBA4404)
- Lysogeny broth (LB) medium (pH 7.0, containing 50 mg/L rifampicin and 50 mg/L kanamycin)
- Murashige and Skoog medium (MS) liquid medium (Sigma-Aldrich, catalog number: M5524 )
- 100 μM acetosyringone (Sigma-Aldrich, catalog number: D134406 )
- 10 mM MgCl2
- 0.005% Silwet L-77 (Agri-Turf Supplies, catalog number: VIS-01 )
- Glycerol
- Filter paper (Lab Depot, catalog number: TLDCFP1-032 )
- Yeast extract broth (YEB) medium (see Recipes)
Equipment
- Vacuum pump (Barnant, model: 400-1903 )
- Desiccator (Ted Pella, catalog number: 2246 )
- Erlenmeyer flasks (VWR International, catalog number: 89095-266 )
Procedure
- Glycerol stocks of A. tumefaciens strain EHA105 (or LBA4404) containing plant expression vector construct were thawed and then streaked onto solid LB medium for 48 h (pH 7.0, containing 50 mg/L rifampicin and vector-corresponding antibiotics such as 50 mg/L kanamycin), then individual single colonies were inoculated into 5 ml of liquid YEB medium containing 50 mg/L rifampicin and 50 mg/L kanamycin.
- These cultures were grown overnight with shaking at 28 °C/200 rpm. The subsequent 100 ml cultures were inoculated with 1 ml of the preceding 5 ml overnight (10-12 h) cultures, and again incubated with shaking at 28 °C/200 rpm until OD600 = 0.5.
- Bacterial cells were harvested by centrifugation at 6,000 x g at 4 °C for 5 min and washed once with 10 ml of washing solution containing 10 mM MgCl2 and 100 μM acetosyringone. After centrifugation at 6,000 x g at 4 °C for another 5 min, the pellet of bacterial cells was resuspended in 50 ml of liquid MS medium.
- Seed sterilization, germination, and growth. Artemisia annua seeds were treated for 1 min in 1 ml of 70% ethanol in a sterile Eppendorf tube. The tube was vortexed and ethanol was then removed. Seeds were subsequently washed four times using aseptic H2O. These surface-sterilized seeds were then treated for 10 min using 10% clorox in a sterile Eppendorf tube, during which the tube was vortexed at least 5 times. Then clorox was removed, seeds were washed four times using aseptic H2O. Sterilized seeds were placed on phytoagar-solidified MS medium, then placed in an incubator with necessary photoperiod and temperature.
- Artemisia annua aseptic seedlings were sub-cultured, after 4-week-growth (Figure 1), the leaves of 7th and 8th internode were chosen and petioles of leaves far from stem were cut before soaked with 50 ml of MS liquid medium containing A. tumefaciens cells supplemented with 0.005% (v/v; i.e. 50 μl/L) surfactant Silwet L-77 in 100 ml sterilized Erlenmeyer flasks, then the Erlenmeyer flasks, covered with a filter to prevent contamination, were placed in the desiccator and a vacuum was applied at 30 inHg for 10 min under the control of a vacuum pump. More than 30 leaves were cut and vacuumed at the same time. The vacuum pump was then switched off and air was slowly let into the desiccator, so as to minimize the damage to the tissues.
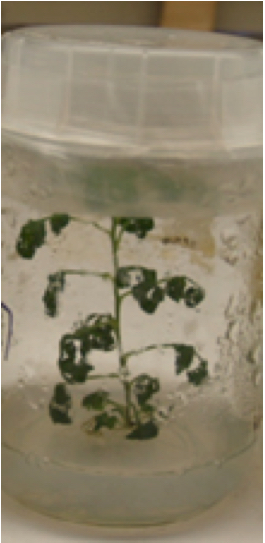
Figure 1. Artemisia annua leaves from 4-week-old aseptic seedlings were used for transient expression, the leaves of 7th and 8th internode were chosen and petiole of leaves far from stem were cut before they were used for transient transformation - Leaves were washed three times by aseptic H2O, then liquid medium was removed using aseptic filter paper, and the explants were plated on MS solid medium for aseptic culture. Usually, when target gene(s) were fused with GUS or GFP maker (Figure 2), the co-cultivation time depends on the target genes expression efficiency, so the co-cultivation was in the dark at 25 °C for 36-72 h.

Figure 2. GUS staining was done using Artemisia annua leaves through transient transformation
Recipes
- Yeast extract broth (YEB) medium
5 g/L beef extract
1 g/L yeast extract
5 g/L peptone
5 g/L sucrose
0.5 g/L MgCl2
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 61173098, No. 61379081).
References
- Han, J., Wang, H., Ye, H., Liu, Y., Li, Z., Zhang, Y., Zhang, Y., Yan, F. and Li, G. (2005). High efficiency of genetic transformation and regeneration of Artemisia annua L. via Agrobacterium tumefaciens-mediated procedure. Plant Sci 168(1): 73-78.
- Ji, Y., Xiao, J., Shen, Y., Ma, D., Li, Z., Pu, G., Li, X., Huang, L., Liu, B., Ye, H. and Wang, H. (2014). Cloning and characterization of AabHLH1, a bHLH transcription factor that positively regulates artemisinin biosynthesis in Artemisia annua. Plant Cell Physiol 55(9): 1592-1604.
- Ma, D., Pu, G., Lei, C., Ma, L., Wang, H., Guo, Y., Chen, J., Du, Z., Wang, H., Li, G., Ye, H. and Liu, B. (2009). Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4,11-diene synthase gene, a key gene of artemisinin biosynthesis. Plant Cell Physiol 50(12): 2146-2161.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ma, D. and Wang, H. (2015). Transient Transformation of Artemisia annua. Bio-protocol 5(10): e1476. DOI: 10.21769/BioProtoc.1476.
Category
Plant Science > Plant transformation > Agrobacterium
Plant Science > Plant physiology > Plant growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link