- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Protein Extraction from Drosophila Embryos and Ovaries
Published: Vol 5, Iss 9, May 5, 2015 DOI: 10.21769/BioProtoc.1459 Views: 20889
Reviewed by: Arsalan DaudiTzvetina BrumbarovaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protein Expression, Purification and Crystallization of the Sxl-Unr-msl2 Ribonucleoprotein Complex
Janosch Hennig and Michael Sattler
Sep 5, 2016 10245 Views

Expression, Purification and Crystallisation of the Adenosine A2A Receptor Bound to an Engineered Mini G Protein
Byron Carpenter and Christopher G. Tate
Apr 20, 2017 12044 Views

A One-Step Method for Efficient Purification of Functional Cas9 Protein
Xinzhi Duan [...] Aihua Mao
Feb 5, 2026 157 Views
Abstract
Here we provide the description of protocols to efficiently obtain protein extracts from embryos and ovaries of Drosophila melanogaster. These protocols are routinely applied in our laboratory and are based on two techniques: either embryos or ovaries are homogenized using a pestle and then the soluble proteins separated by centrifugation, or embryos are individually lysed by needle manipulation. The latter technique allows the use of small embryo numbers and the selection of specific developmental stages (Guilgur et al., 2014).
Keywords: DrosophilaMaterials and Reagents
- Phosphate buffered saline tablets (Sigma-Aldrich, catalog number: P4417-100TAB )
- Commercial bleach solution
- Tween 20 (Sigma-Aldrich, catalog number: P5927 )
- Tris-base (DBH Prolabo, catalog number: 33621.26 0)
- NaCl (Panreac Applichem, catalog number: 121659.1211 )
- EDTA (Sigma-Aldrich, catalog number: E6758 )
- DL-dithiothreitol (DTT) (Sigma-Aldrich, catalog number: 43819 )
- NP-40 (IGEPAL CA-630) (Sigma-Aldrich, catalog number: I8896 )
- Sodium fluoride (NaF) (Fluka, catalog number: 71519 )
- NaOH (sodium hydroxide pellets) (Panreac, catalog number: 131687 )
- Agar-agar (Nzytech, Agar-agar, catalog number: MB14801 )
- Sugar (commercial)
- Apple juice (commercial)
- Niapagine (Dutscher, Niapagine, catalog number: 789063 )
- Complete EDTA-free protease inhibitor tablets (Roche Diagnostics, catalog number: 04693159001 )
- Sample buffer (2x Laemmli sample buffer) (Sigma-Aldrich, catalog number: S3401 )
- Commercial fresh baker´s yeast paste
- NB lysis buffer (see Recipes)
- 1 M Tris-HCl (see Recipes)
- 500 mM EDTA (see Recipes)
- 10% NP-40 (see Recipes)
- 0.5 M NaF (see Recipes)
- 1 M DTT (see Recipes)
- Apple juice plates (see Recipes)
Equipment
- Containers (dark tip boxes to increase the contrast with the white embryos)
- Cell strainer (70 μm nylon cell strainer) (BD Biosciences, Falcon®, catalog number: 352350 )
- 1.5 ml tubes
- 1.5 μl pestles (Kimble Chase, catalog number: 749521-1590 )
- Paint brush number 4
- Needles (0.8 x 25 mm) (Terumo, catalog number: NN-2125R )
- Tweezers (Fine Science Tools, Dumont #5 )
- Refrigerated centrifuge (Eppendorf, model: 5424R )
- Fly cages
- Small Petri dishes (Sarstedt, catalog number: 83.1801.002 )
- Filters (Acrodisc Syringe Filters 0.2 μm Supor Membrane) (Pall, catalog number: PN 4612 )
Procedure
- For protein extraction from ovaries
- Ovary dissection
- Rear female flies alongside a small fraction (1:3) of males in food supplemented with fresh baker yeast paste for one to two days prior to dissection. This will stimulate oogenesis and lead to bigger ovaries (with increased numbers of late developmental stages).
- Inactivate anaesthetized flies by decapitation.
- Dissection technique (under stereoscope and using dissection plate and a pair of tweezers): For each fly, place the organism in a drop of 1x PBS and hold it in place by applying gentle pressure at the level of the upper thorax. Using the tweezers in the free hand tug gently at the lower part of the abdomen (ovipositor region) until the cuticle starts to detach from the fly, exposing the internal organs. Isolate ovaries from adjoining tissues and organs and transfer them to ice cold 1x PBS while dissecting the remaining flies (avoid keeping the ovaries in the 1x PBS solution for periods longer than 30 min) (see Video 1 for a visual description). Video 1. Drosophila ovary dissection
- Rear female flies alongside a small fraction (1:3) of males in food supplemented with fresh baker yeast paste for one to two days prior to dissection. This will stimulate oogenesis and lead to bigger ovaries (with increased numbers of late developmental stages).
- Ovary protein extraction by sample homogenization
- Transfer the isolated ovaries to a 1.5 ml tube containing 200 μl of ice-cold NB lysis buffer.
- Manually homogenize samples using a pestle. Homogenization should ensure the complete breakdown of the tissue. If pestles are to be reused, wash them thoroughly with distilled water before processing other samples.
- Centrifuge for 20 sec at ~10,000 rcf (4 °C) to settle down at the bottom of the tube the unprocessed tissue.
- Repeat manual homogenization of the centrifuged material.
- Centrifuge for 3 min at ~20,000 rcf (4 °C).
- Transfer the supernatant to a new 1.5 ml tube, avoiding the upper lipid layer.
- Repeat this centrifugation process (steps A2 e-f) two more times.
- Quantify protein concentration and dilute to the final concentration (dependent on the requirements of downstream applications).
- Dilute final concentration with an equal volume of 2x Laemmli sample buffer.
- Heat samples for 5 min at 100 °C and immediately freeze them at -20 °C after a quick centrifuge spin-down. Extracts can be stored at -20 °C until necessary.
- Transfer the isolated ovaries to a 1.5 ml tube containing 200 μl of ice-cold NB lysis buffer.
- Ovary dissection
- For protein extraction from embryos
- Embryo collection and processing
- For one to two days prior to embryo collection rear male and female flies on collection cages with standard apple juice agar plates supplemented with fresh baker yeast paste (Figure 1 A).
- Start the collection by placing a clean apple juice agar plate on the cage. Let flies lay eggs for a given time interval.
- While egg laying is taking place prepare the 5 individual containers for the subsequent processing of the embryos. Containers with the following solutions are required: 0.1% Tween 20 (in water), 50% commercial bleach (in water) and deionized water (3 containers). Place a collection basket (cell strainer) into the container with the 0.1% Tween 20 solution (starting point) (Figure 1A).
- Collect embryos from the agar plate using a small paintbrush and place them in the partially immersed basket.
- Gently stir the collection basket to wash the embryos. Dry the base of the collection basket in a paper tissue before transferring it to the subsequent container.
- Transfer the embryos in the collection basket to the container with the 50% commercial bleach solution. Incubate for 5 min with gentle, periodic stirring. The purpose of this step (dechorionation) is to remove the chorionic membranes which constitute the eggshell covering the embryos. The dechorionated embryos will become hydrophobic and will float on the surface of the bleach solution (Figure 1B).
- Transfer the embryos in the collection basket to a new container with deionized water. Wash for 2 min and repeat twice using each time new containers. Before starting the washes and also when transferring between the water containers dry the base of the collection basket in a paper tissue.
Note: Embryos can be stored at this step by transferring them to a 1.5 ml tube (remove excess water) that will be flash frozen in liquid nitrogen prior to storage at -80 °C.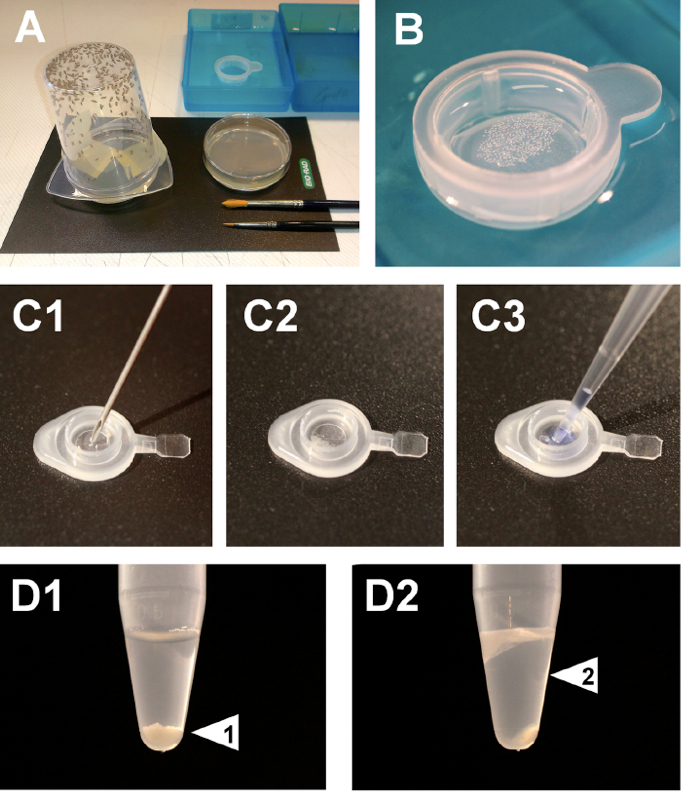
Figure 1. Set up for embryonic protein extraction. Fly collection cages with standard apple juice agar plates and solution containers set up (A). Dechorionated embryos float on the surface of the bleach solution (B). Manually select embryos collected in a 0.5 ml tube lid (C1). Punctured embryo total extract (C2). Total extract collection by mixing with Laemmli sample buffer (C3). Embryo collection before pestle homogenization (Arrowhead 1, D1). Embryonic soluble protein extract after homogenization and centrifugation (Arrowhead 2, D2).
- For one to two days prior to embryo collection rear male and female flies on collection cages with standard apple juice agar plates supplemented with fresh baker yeast paste (Figure 1 A).
- Embryo total protein extracts
Manual selection and needle homogenization protocol- After dechorionation, manually select embryos with sharp tweezers or a fine paintbrush and transfer them to a previously sectioned 0.5 ml tube lid (for a minimum of 10 embryos per lid) (Figure 1C1).
- Dry as much as possible the embryos by absorbing the excess water with a dry paintbrush.
- Individually puncture each embryo using a needle (Figure 1C2).
- Gently mix the resulting lysate with 10 µl of 1x Laemmli sample buffer (Figure 1C3). Transfer the solution to a 1.5 ml collection tube.
Note: The volume of sample Laemmli buffer is according to the number of embryos used (1 µl per embryo). - Heat samples for 5 min at 100 °C and immediately freeze them at -20 °C after a quick centrifuge spin-down. Extracts can be stored at -20 °C until necessary.
Pestle homogenization protocol- After dechorionation, transfer embryos (20 μl volume) to a 1.5 ml tube containing 200 μl of ice-cold NB lysis buffer (Figure 1D1).
Note: This will correspond approximately to between 1 to 2 μg/μl of final protein concentration. Manually homogenize embryos using a pestle (~10 strokes). Homogenization should ensure the complete breakdown of the tissue. If pestles are to be reused, wash them thoroughly before processing other samples. - Centrifuge for 20 sec at ~10,000 rcf (4 °C) to settle down at the bottom of the tube the unprocessed tissue.
- Repeat manual homogenization of the centrifuged material.
- Centrifuge for 3 min at ~20,000 rcf (4 °C).
- Transfer the supernatant to a new 1.5 ml tube, avoiding the upper lipid layer (Figure 1D2).
- Repeat this centrifugation process (steps B2 i-j) two more times.
- Quantify protein concentration and dilute to the final concentration (dependent on the requirements of downstream applications).
- Dilute final concentration with an equal volume of 2x Laemmli sample buffer.
- Heat samples for 5 min at 100 °C and immediately freeze them at -20 °C after a quick centrifuge spin-down. Extracts can be stored at -20 °C until necessary.
- After dechorionation, manually select embryos with sharp tweezers or a fine paintbrush and transfer them to a previously sectioned 0.5 ml tube lid (for a minimum of 10 embryos per lid) (Figure 1C1).
- Embryo collection and processing
Recipes
- NB bufferAdd ddH2O to final volume of 50 ml
Initial concentration Volume Final concentration 1 M NaCl 7.5 ml 150 mM NaCl 1 M Tris-HCl (pH 7.5) 2.5 ml 50 mM Tris-HCl (pH 7.5) 500 mM EDTA (pH 8.0) 200 µl 2 mM EDTA 10% NP-40 500 µl 0.1% NP-40
Sterilized by filtration (0.2 µm filter)
Make 10 ml Aliquots and store at -20 °C
Before use add to the 10 ml aliquot: 10 µl of 1 M DTT, 200 µl of 0.5 M NaF and dissolve one Complete EDTA-free tablet to the solution - 1 M Tris-HCl (pH 7.5)
Dissolve 157.6 g of Tris-HCl to ~800 ml of ddH2O
Adjust pH to 7.5 with NaOH
Add ddH2O to final volume of 1,000 ml
Sterilized by filtration (0.2 µm filter)
Stored at RT - 500 mM EDTA (pH 8.0)
Weigh 73.06 g of EDTA to ~400 ml of ddH2O
Adjust pH slowly to 8.0 with NaOH - EDTA dissolves when pH approaches 8
Add ddH2O to final volume of 500 ml
Sterilized by filtration (0.2 µm filter)
Stored at RT - 10% NP-40
Dilute 10 ml of NP-40 to ddH2O in a final volume of 100 ml
Sterilized by filtration (0.2 µm filter)
Stored at RT - 0.5 M NaF
Dissolve 2.0995 g of NaF to ddH2O in a final volume of 100 ml
Sterilized by Filtration (0.2 µm filter)
Aliquot and stored at -20 °C - 1 M DTT
Dissolve 1.5425 g of DTT to ddH2O in a final volume of 10 ml in the fume hood
Sterilized by filtration (0.2 µm filter)
Aliquot and stored at -20 °C - Apple juice plates (1 L)
Weigh Agar-agar in a big plastic beaker 19.5 g
Add to the Agar-agar ddH2O 500 ml
Mix everything very well
Place the beaker in microwave until boiling
Wait for the medium to cool to 50 °C, stirring from time to time to avoid the formation of a film on the surface
Weigh sugar in an aluminum foil 20 g
Then add to the dissolve Agar-agar: The sugar, Apple juice 250 ml, Niapagin 10% 5 ml and ddH2O 250 ml
1 L of apple juice medium → 100 small plates
Store the plates at 4 °C no more than 30 days
Acknowledgments
We like to thank Paulo Navarro-Costa for critical reading of manuscript and Rui Martinho for his supervi-sion. Funding: FCT-Fundaçao para a Ciencia e Tecnologia (Portugal): Leonardo Gastón Guilgur, SFRH/BPD/47957/2008. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
References
- Guilgur, L. G., Prudencio, P., Sobral, D., Liszekova, D., Rosa, A. and Martinho, R. G. (2014). Re-quirement for highly efficient pre-mRNA splicing during Drosophila early embryonic development. Elife 3: e02181.
Article Information
Copyright
Prudêncio and Guilgur. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Prudêncio, P. and Guilgur, L. G. (2015). Protein Extraction from Drosophila Embryos and Ovaries. Bio-protocol 5(9): e1459. DOI: 10.21769/BioProtoc.1459.
- Guilgur, L. G., Prudencio, P., Sobral, D., Liszekova, D., Rosa, A. and Martinho, R. G. (2014). Re-quirement for highly efficient pre-mRNA splicing during Drosophila early embryonic development. Elife 3: e02181.
Category
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











