- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Glioma Associated Stem Cells (GASCs) Isolation and Culture
Published: Vol 5, Iss 9, May 5, 2015 DOI: 10.21769/BioProtoc.1457 Views: 10150
Reviewed by: Hui ZhuSalma HasanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation of Stem Cells, Endothelial Cells and Pericytes from Human Infantile Hemangioma
Lan Huang and Joyce Bischoff
Jan 20, 2020 4962 Views

Low-viscosity Matrix Suspension Culture for Human Colorectal Epithelial Organoids and Tumoroids
Tao Tan [...] Oliver M. Sieber
Apr 20, 2022 4058 Views

Thrombopoietin-independent Megakaryocyte Differentiation of Hematopoietic Progenitor Cells from Patients with Myeloproliferative Neoplasms
Chloe A. L. Thompson-Peach [...] Daniel Thomas
Jan 20, 2023 2363 Views
Abstract
Glioma Associated Stem Cells (GASCs) represent a population of non-tumorigenic multipotent stem cells hosted in the microenvironment of human gliomas. In vitro, these cells are able, through the release of exosomes, to increase the biological aggressiveness of glioma-initiating cells. The clinical importance of this finding is supported by the strong prognostic value associated with the GASCs surface immunophenotype thus suggesting that this patient-based approach can provide a groundbreaking method to predict prognosis and to exploit novel strategies that target the tumor stroma (Bourkoula et al., 2014).
Keywords: Human gliomaMaterials and Reagents
- Hank’s balanced salt solution (HBSS) without calcium and magnesium (Sigma-Aldrich, catalog number: H2387 )
- Collagenase type II (Sigma-Aldrich, catalog number: C6885-500MG )
- Fetal bovine serum (FBS) (EuroClone, catalog number: ECS0180L )
- Trypan blue (Sigma-Aldrich, catalog number T6146-5G )
- Dulbecco’s phosphate buffered saline (D-PBS) (Life Technologies, catalog number: 14190-250 )
- Fibronectin from human plasma 0.1% solution (cell culture tested) (Sigma-Aldrich, catalog number: F0895 )
- Modified Eagle’s medium (MEM) Joklik without NaHCO3 (Sigma-Aldrich, catalog number: M0518 )
- Hepes (powder) (Sigma-Aldrich, catalog number: H3375 )
- L-glutamine (Sigma-Aldrich, catalog number: G7513 )
- Taurine (Sigma-Aldrich, catalog number: T0625 )
- Penicillin-streptomycin (100x solution stabilized, sterile-filtered, suitable for cell culture) (Sigma-Aldrich, catalog number: P4333-100ML )
- Insulin (Sigma-Aldrich, catalog number: I5523-50MG )
- Dulbecco’s modified Eagle’s medium (DMEM) low glucose (Life Technologies, Gibco®, catalog number: 31600-091 )
- MCDB-201 (Sigma-Aldrich, catalog number: M6770 )
- Linoleic acid-bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: L9530 )
- Dexamethasone (powder) (Sigma-Aldrich, catalog number: D2915 )
- Ascorbic acid-2 phosphate (powder) (Sigma-Aldrich, catalog number: A8960 )
- 100x insulin-transferrin-selenium (ITS) (Life Technologies, Gibco®, catalog number: 41400-045 )
- Stem cell tested fetal bovine serum (Stem Cells Technologies, catalog number: 10M37180 )
- Human platelet derived growth factor-BB (hPDGF-BB) (Pepro Tech, catalog number: 100-14B )
- Human epidermal growth factor (hEGF) (Pepro Tech, catalog number: AF-100-15 )
- TrypLE-express dissociation reagent (Life Technologies, Gibco®, catalog number: 12605 )
- Basic buffer (BB) (see Recipes)
- Incubation buffer (IB) (see Recipes)
- 0.025% Collagenase type II (see Recipes)
- 0.4% Trypan blue solution (see Recipes)
- Fibronectin-coated 100 mm petri dish (see Recipes)
- Multipotent adult stem cell (MASC) medium (see Recipes)
Equipment
- Sterile tissue culture dishes 100 x 20 mm style (BD Biosciences, Falcon®, catalog number: 353003 )
- Scalpel blades (Sigma-Aldrich, catalog number 27046 )
- Sterile serological transfer pipettes
- Serological pipettes
- Sterile Falcon tubes
- Mesh filter (70 µm) (BD Biosciences, Falcon®, catalog number 352350 )
- Biological hood (Faster, model: Safe FAST Elite )
- Agitation rotor (Celbio, model: MINI OVEN )
- Burker counting chamber (Sigma-Aldrich, catalog number: Z359629-1EA )
- 37 °C, 5% CO2, 5% O2 force air incubator (New Brunswick, model: Galaxy 170 R )
- Centrifuge (Heraeus Instruments, model: Megafuge 1.0 R )
- Light microscope (Leica Microsystems, model: DMIL )
Procedure
- Isolation and primary culture of primitive cells from human glioma (see Video 1)
- Under biological hood, place the glioma sample into sterile 100 mm Petri dish and cut the fragments into ~1 mm pieces using scalpels.
- Re-suspend glioma fragments into 3-5 ml of BB and transfer them into a 15 ml Falcon tube.
- Let the fragments deposit to the bottom of the 15 ml Falcon tube by gravity at room temperature. If fragments will not deposit into the bottom, centrifuge the suspension at 300 x g for 1 min.
- Remove the supernatant and re-suspend the volume of the pellet in an equal volume of collagenase solution (0.025%).
- Incubate the suspension for 5 min in an agitation rotor at 37 °C.
- At the end of the incubation period, gently shake the bottom of the Falcon tube and add 5 ml of IB and gently pipette it with a 10 ml serological pipette for 4-5 min.
- Pre-wet a 70 μm mesh filter by filtering 5 ml of sterile, fresh HBSS using a 5 ml sterile serological pipette into a 50 ml Falcon tube.
- Filter the cell suspension through the pre-wet 70 μm mesh filter.
- Centrifuge the filtered suspension at 500 x g for 5 min at room temperature and re-suspend it into 1 ml of MASC medium.
- Count the cells using the Burker Counting chamber: Remove 10 μl of cell suspension, mix it with an equal volume of 0.4% Trypan blue in D-PBS and apply 10 μl of diluted cells to the Burker chamber. Count at least four squares and calculate the number of cells by multiplying the medium value obtained for 2 x 104. You will obtain the total amount of cells/ml of cell suspension.
- Seed 5,000 cells/cm2 into fibronectin-coated dishes and culture them in MASC medium at 37 °C in a 5% CO2, 5% O2 force air incubator.
- Under biological hood, place the glioma sample into sterile 100 mm Petri dish and cut the fragments into ~1 mm pieces using scalpels.
- Sub-culture of glioma associated stem cells (GASCs)
- Take out from the incubator (5% CO2, 5% O2, at 37 °C) the dish containing the cells that has to be split. Check at the microscope that cells have reached 80% of confluence (7-10 days after seeding).
- Transfer the dish below the biological hood, open the dish and discard the supernatant with a 10 ml sterile serological pipette.
- Add 5 ml of HBSS or D-PBS to the dish, gently move the plate in order to cover the entire surface, and then remove the HBSS or D-PBS. Repeat the washing procedure. Remove carefully the HBSS or D-PBS.
- Add 2 ml of TrypLE Express solution for each dish. Since cells will detach very fast, check continuously at the microscope the process in order to avoid over-digestion.
- When cells are finally detached, add 5 ml of HBSS or D-PBS to dilute the TrypLE Express solution.
- Re-suspend the cells by gently pipetting them through a 10 ml sterile serological pipette. To recover all cells from the dishes, wash the Petri dish one more time, by adding 3 ml of fresh sterile HBSS or D-PBS and put the solution in the Falcon Tube containing the previous obtained cell suspension.
- Centrifuge the cell suspension at 500 x g for 5 mins at room temperature, discard the supernatant and re-suspend the cell pellet in 1 ml of MASC medium.
- Seed the cells onto fibronectin-coated dishes at the concentration of 3,500 cells/cm2.
- Change the medium every three days. Split the cells when they will reach 80% confluence.
- Take out from the incubator (5% CO2, 5% O2, at 37 °C) the dish containing the cells that has to be split. Check at the microscope that cells have reached 80% of confluence (7-10 days after seeding).
Representative data
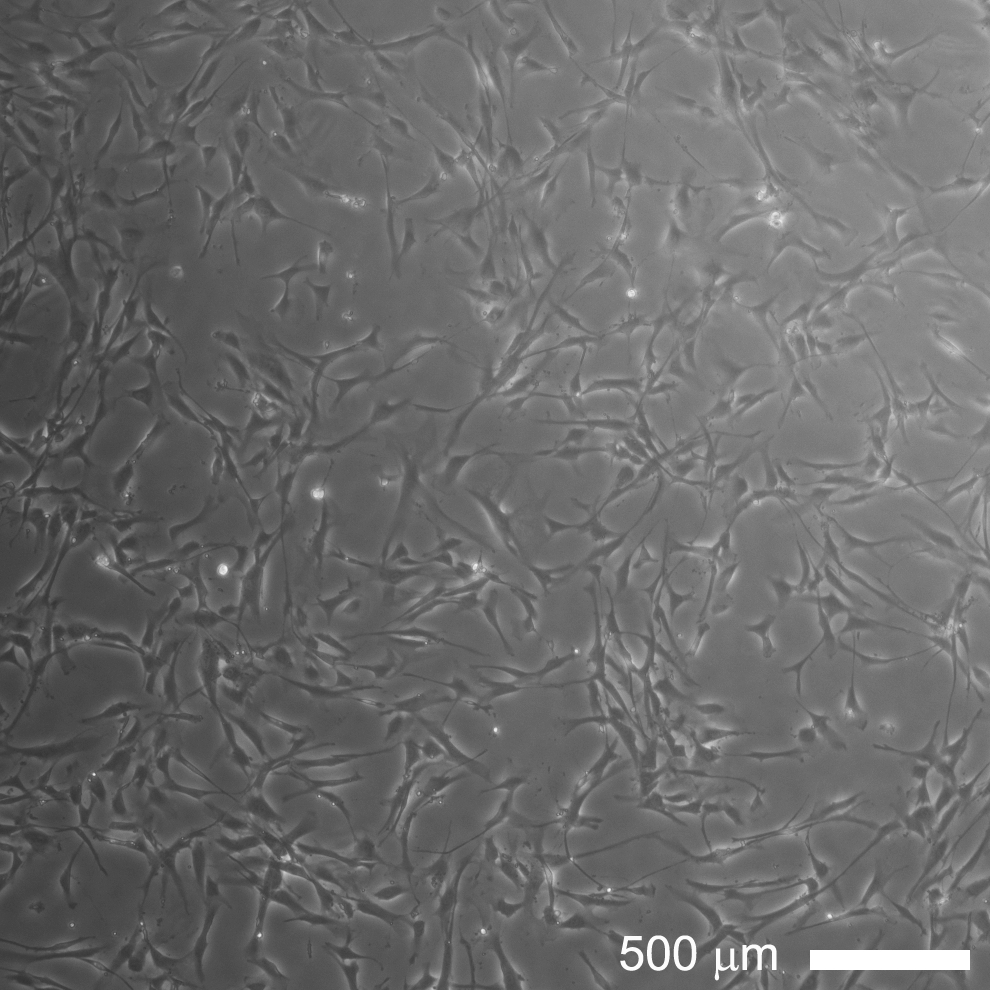
Figure 1. Phase contrast image of GASCs at the third passage in culture
Recipes
- Basic buffer (BB)
11 g MEM Joklik without NaHCO3 (powder)
4.7 g Hepes (powder)
0.3 g L-glutamine
0.25 g taurine
5 ml penicillin-streptomycin
Insulin 20 U/L
Add non-pyrogenic dH2O up to 900 ml and adjust the pH to 7.3
Add non-pyrogenic dH2O up to 1,000 ml and filter sterilize (0.2 µm)
Stored at 4 °C - Incubation buffer (IB)
Add 10% of fetal bovine serum to HBSS
Stored at 4 °C - 0.025% Collagenase type II
0.025 g Collagenase type II
Add 10 ml of BB and carefully pipette for 2-3 min
Add BB up to 100 ml, filter sterilize and stored at -20 °C - 0.4% Trypan blue solution
0.1 g Trypan blue
Add 25 ml sterile 1x D-PBS
Vortex and stored at room temperature - Fibronectin-coated 100 mm petri dish
For each 100 mm petri dish, add 5 µl 0.1% fibronectin in 1 ml of HBSS
Carefully cover the bottom of the dish with the fibronectin solution and keep for at least 20 min at room temperature
Remove the exceeding solution from the dish - Multipotent adult stem cell (MASC) medium
Base medium (60% DMEM low glucose + 40% MCDB-201)
2% stem cell tested fetal bovine serum
10 ng/ml hPDGF-BB
10 ng/ml hEGF
1 mg/ml linoleic acid-BSA
10-9 M dexamethasone
1x ITS 100x
10-4 M ascorbic acid-2 phosphate
1x penicillin-streptomycin 100x
Adjust the pH to 7.3
Filter sterilize (0.2 µm) and stored at 4 °C
Acknowledgments
FIRB Accordi di programma 2011 Pr. RBAP11Z4Z9. FIRB accordi di programma 2011 Pr. RBAP11ETKA_007. Programma per la Cooperazione Transfrontaliera Italia-Slovenia 2007-2013. Title: “Identificazione di nuovi marcatori di cellule staminali tumorali a scopo diagnostico e terapeutico”; AIRC 5 per mille Special program 2011, Pr. 12214. Project ERC- 7FP SP 2 IDEAS QUIDPROQUO G.A. n. 269051. The protocol was adopted from Bourkoula et al. (2014).
References
- Andolfi, L., Bourkoula, E., Migliorini, E., Palma, A., Pucer, A., Skrap, M., Scoles, G., Beltrami, A. P., Cesselli, D. and Lazzarino, M. (2014). Investigation of adhesion and mechanical properties of human glioma cells by single cell force spectroscopy and atomic force microscopy. PLoS One 9(11): e112582.
- Beltrami, A. P., Cesselli, D., Bergamin, N., Marcon, P., Rigo, S., Puppato, E., D'Aurizio, F., Verardo, R., Piazza, S. and Pignatelli, A. (2007). Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow). Blood 110(9): 3438-3446.
- Bourkoula, E., Mangoni, D., Ius, T., Pucer, A., Isola, M., Musiello, D., Marzinotto, S., Toffoletto, B., Sorrentino, M., Palma, A., Caponnetto, F., Gregoraci, G., Vindigni, M., Pizzolitto, S., Falconieri, G., De Maglio, G., Pecile, V., Ruaro, M. E., Gri, G., Parisse, P., Casalis, L., Scoles, G., Skrap, M., Beltrami, C. A., Beltrami, A. P. and Cesselli, D. (2014). Glioma-associated stem cells: a novel class of tumor-supporting cells able to predict prognosis of human low-grade gliomas. Stem Cells 32(5): 1239-1253.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bourkoula, E., Mangoni, D., Caponnetto, F., Ius, T., Skrap, M., Beltrami, A. P. and Cesselli, D. (2015). Glioma Associated Stem Cells (GASCs) Isolation and Culture. Bio-protocol 5(9): e1457. DOI: 10.21769/BioProtoc.1457.
Category
Stem Cell > Adult stem cell > Cancer stem cell
Cancer Biology > Cancer stem cell > Cell biology assays > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










