- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measuring Arabidopsis, Tomato and Barley Leaf Relative Water Content (RWC)
Published: Vol 5, Iss 8, Apr 20, 2015 DOI: 10.21769/BioProtoc.1451 Views: 16126
Reviewed by: Tie LiuXiao-qing Xu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Simple Sonication Method to Isolate the Chloroplast Lumen in Arabidopsis thaliana
Jingfang Hao and Alizée Malnoë
Aug 5, 2023 2306 Views

A Plate Growth Assay to Quantify Embryonic Root Development of Zea mays
Jason T. Roberts [...] David M. Braun
Oct 20, 2023 2271 Views

Detection and Quantification of Programmed Cell Death in Chlamydomonas reinhardtii: The Example of S-Nitrosoglutathione
Lou Lambert and Antoine Danon
Aug 5, 2024 1605 Views
Abstract
Measuring leaf relative water content (RWC) is a reliable and simple way to assess the water status of a leaf without any need for special equipment. Similar to leaf water potential, leaf RWC gives a strong indication of the plant’s response to different environmental conditions; yet RWC has been shown to be a more stable parameter than leaf water potential (Sade et al., 2009; Sade et al., 2012). Although measuring RWC is destructive to the leaf, with proper planning, it need not affect the plant’s behavior. This note will focus on three different model plants which are representative of plants with various leaf shapes (e.g., Arabidopsis, tomato and barley). The technique for measuring RWC is the same for all three of these species (as well as for plants with many other types of leaves).
Keywords: Relative water contentMaterials and Reagents
- 4-week-old Arabidopsis plants grown under short-day conditions (8/16 L/D; 22/22 °C)
- 4-week-old tomato plants grown under moderate conditions (10/14 L/D; 25/20 °C)
- 4-week-old barley plants grown under short-day conditions (8/16 L/D; 16/10 °C)
Equipment
- Zipper-locked plastic bag (8 x 12 cm) for each sample
- Paper bag (8 x 12 cm) for each sample
- Sharp scissors
- Scalpel
- Paper towels
- Analytical balance with 0.1 mg readability
- 2-3 ml of 5 mM CaCl2 in DDW water for each sample
- Marker pen
- Plastic box that can be sealed tightly
Software
- Microsoft Excel
Procedure
- Grow the plants.
- Mark and weigh each plastic bag (BW) prior to the other measurements.
- Typically, leaves are measured before dawn and/or at midday (Matin et al., 1989). At the selected hour, select a fully expanded and mature leaf.
- For Arabidopsis and tomato, cut the leaf with a scalpel leaving a 1-cm-long petiole.
- For barley (or any other leaf without a petiole; e.g., rice, wheat), use scissors to cut a 6- to 10-cm-long piece of leaf blade. It is important to harvest leaf pieces of the same size and from the same area (typically 6-10 cm starting from the leaf tip). It is recommended to use at least 4-5 biological replications especially when measuring plants under different treatments (e.g. drought stress).
- Place the harvested leaf in a plastic bag immediately after cutting and close the bag (Figure 1).

Figure 1. Representative leaves in the Zipper-locked plastic bag (8 x 12 cm). A. Arabidopsis mature rosette leaf; B. Tomato mature fully expanded leaflet; C. Barley 6- to 10-cm-long leaf blade end. Bottom and right part of the pic show a roller for estimation of plastic bag size. - Make sure the bag is tightly sealed so that no vapor can escape. Put the bag in the closed (dark) box.
- Weigh each bag with the fresh leaf inside to determine the total fresh weight (TFW).
- Open the bag and gently make sure that the petiole is facing down. Insert 2-3 ml of 5 mM CaCl2 into the bag and make sure that only the leaf petiole is immersed in the solution (Figure 2). Close the bag and put the sample back in the box at room temperature.
- After 8 h, take the leaf out of the bag and put it between two paper towels to absorb excess water.
- Weigh the turgid leaf to determine the turgid weight (TW).
- Insert each sample into a paper bag and dry in a 60 °C dry oven for 3-4 days.
- Weigh the dried samples to determine the dry weight (DW).
- Calculate the relative water content of the leaf:
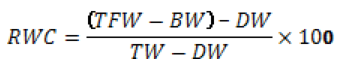
Notes
- It is very important to use high-quality zipper-locked bags, to prevent humidity and liquids from leaking out of the bag.
- Work quickly, but gently to maintain reliable results.
- Maintain the same order of sampling to ensure that all samples are measured using the same protocol.
- There are different zipper-locked bags that fit leaves of different sizes. Choose bags of a size appropriate for the species with which you are working.
- Make sure that only the petiole is immersed in the solution (or the cut part of the leaf in the case of grass species; Figure 2).

Figure 2. Leaves in the Zipper-locked plastic bag after insertion of 2-3 ml of 5 mM CaCl2 into the bag. A. Arabidopsis mature rosette leaf; B. Tomato mature fully expanded leaflet; C. Barley 6- to 10-cm-long piece of leaf blade.
Note the solution in the bottom part of the plastic bag. - This protocol was used for measurements of 4 weeks old Tomato, Arabidopsis and Barley leaves under well irrigated conditions and short day photoperiod as stated in the Materials and Reagents section. However, it is suitable for different physiological age (as long as the leaves are fully expanded), different plants (as long as the morphological shape of the leaves is similar to those appear in this protocol), different photoperiod (both long and short day) and different irrigation regime (drought and salt).
Acknowledgments
This work was supported by the Israel Science Foundation (grant no. 1131/12) and the German-Israeli Project Cooperation (grant nos. FE 552/12–1 to A.R.F. and OR309/1-1.
This Protocol was adapted from Sade et al. (2014).
References
- Matin, M., Brown, J. H. and Ferguson, H. (1989). Leaf water potential, relative water content, and diffusive resistance as screening techniques for drought resistance in barley. Agron J 81(1): 100-105.
- Sade, N., Gebremedhin, A. and Moshelion, M. (2012). Risk-taking plants: anisohydric behavior as a stress-resistance trait. Plant Signal Behav 7(7): 767-770.
- Sade, D., Sade, N., Shriki, O., Lerner, S., Gebremedhin, A., Karavani, A., Brotman, Y., Osorio, S., Fernie, A. R., Willmitzer, L., Czosnek, H. and Moshelion, M. (2014). Water balance, hormone homeostasis, and sugar signaling are all involved in Tomato resistance to Tomato yellow leaf curl virus. Plant Physiol 165(4): 1684-1697.
- Sade, N., Vinocur, B. J., Diber, A., Shatil, A., Ronen, G., Nissan, H., Wallach, R., Karchi, H. and Moshelion, M. (2009). Improving plant stress tolerance and yield production: is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol 181(3): 651-661.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sade, N., Galkin, E. and Moshelion, M. (2015). Measuring Arabidopsis, Tomato and Barley Leaf Relative Water Content (RWC). Bio-protocol 5(8): e1451. DOI: 10.21769/BioProtoc.1451.
- Sade, D., Sade, N., Shriki, O., Lerner, S., Gebremedhin, A., Karavani, A., Brotman, Y., Osorio, S., Fernie, A. R., Willmitzer, L., Czosnek, H. and Moshelion, M. (2014). Water balance, hormone homeostasis, and sugar signaling are all involved in Tomato resistance to Tomato yellow leaf curl virus. Plant Physiol 165(4): 1684-1697.
Category
Plant Science > Plant physiology > Abiotic stress
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









