- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Gravitropic Analysis of Tomato Seedlings using Time Lapse Video Imaging
Published: Vol 5, Iss 7, Apr 5, 2015 DOI: 10.21769/BioProtoc.1443 Views: 9362
Reviewed by: Tie LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1959 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1897 Views

ClearDepth Method for Evaluations of Root Depth in Soil-Filled Pots
Michel Ruiz Rosquete [...] Wolfgang Busch
Aug 20, 2025 2129 Views
Abstract
Plants use gravity as a guide for growth and development. Gravitropism, a gravity-directed growth process, directs upward shoot movement for efficient photosynthesis and gaseous exchange. In addition, it also directs downward growth of roots in soil, for assimilation of water and nutrients required for growth and development. Using time lapse video imaging this process can be efficiently studied in a real time scale. The analysis of the response under different conditions can help to unravel the mechanisms regulating gravitropism.
Keywords: TomatoMaterials and Reagents
- Tomato seeds (Cultivar Arka Vikas)
- Sodium hypochlorite (Thermo Fisher Scientific, catalog number: 27096 )
- Agar (HIMEDIA, catalog number: PCT0901 )
- Distilled water
- 0.8% (w/v) agar (see Recipes)
Equipment
- Blue light (An alternate light source can also be used) for gravitropic analysis was obtained from blue LEDs (Light Emitting diodes, λmax 470 nm, Kwality Photonics). Photon fluence of light was measured with a light meter fitted with a Quantum sensor (Skye Instruments, UK).
- Spectrum of the blue light was measured using spectroradiometer purchased from International Light Technologies (model: RPS900W)
- Quickcam Pro 3000 or 4000 (Logitech) or any other suitable webcam for recording the response
- Germination paper (locally purchased) or blotting paper or Whatman paper
- Square germination boxes (dimension 10 x10 x 6 cm, Laxbro) / or square petriplates
- Square petriplate (dimension 12x12x2 cm, Genaxy, model: GEN-PTD-130SQ)
- Forceps from Pro’Kit® 1PK-125T (dimension 12 x1 x 4 cm)
Software
- SigmaPlot 10.0 (http://www.sigmaplot.com/products/sigmaplot/sigmaplot-details.php)
- ImageJ (http://imagej.nih.gov/ij/)
- Timelapse (http://tnlsoftsolutions.com/timelapsehome.php)
Procedure
- Plant growth conditions
- Tomato (Solanum lycopersicum L. cultivar Arka Vikas) seeds (Figure 1A) were surface sterilized in 20% (v/v) sodium hypochlorite for about 15 min followed by washing in running tap water to remove any trace of hypochlorite (this step can be performed preferably in a fume hood).
- The sterilized seeds were sown on two layers of wet filter paper in germination box (Figure 1B). (It is better to sterilize box and papers.)
- The seeds were germinated for 3 days in germination boxes (25 ± 2 °C).
- After emergence of the radical (Figure 2), equal sized seedlings were gently transferred on 0.8% (w/v) agar in petriplate and kept in darkness at 25 ± 2 °C for 2 days.
- Unless otherwise mentioned, for all experiments the seedling’s age was counted from the time of emergence of the radical. In all cases the average height of the etiolated seedlings used was 3.0 ± 0.3 cm (Figure 3).
- Tomato (Solanum lycopersicum L. cultivar Arka Vikas) seeds (Figure 1A) were surface sterilized in 20% (v/v) sodium hypochlorite for about 15 min followed by washing in running tap water to remove any trace of hypochlorite (this step can be performed preferably in a fume hood).
- Analysis of gravitropism/gravistimulation
- The gravitropic response of tomato seedlings was examined under omnilateral blue light (white light can also be used). To eliminate any phototropic effect of light on seedlings the time lapse imaging can be done using Infra-red LEDs as a light source. Figure 4 shows the placement of camera and seedlings. Seedlings were placed at a horizontal distance of 20 cm from the camera lens. The light source was 20 cm above the seedlings.
- Gravity response in the hypocotyls was measured using 2-d-old etiolated seedlings, grown in square petriplates filled with 0.8% (w/v) agar (Figure 5A).
- At the time of gravistimulation, plates were turned at an angle of 90° to make the seedlings horizontal, and the plates were arranged in a rack (Figure 5B).
- Seedlings were photographed using a Quickcam Pro 3000 at every 10-min interval for 120 minutes for hypocotyl gravitropism. Figure 5C shows the hypocotyl bending after 120 min of blue light exposure.
- For each experiment, 10 seedlings were used and the experiment was repeated 3 to 4 times.
- For root gravitropism, seeds with just emerged radicles were sown on 0.8% (w/v) agar in petri plates and plates were kept vertically under darkness (as above). After 36 h, when roots elongated to ca. 2 to 2.5 cm, the plates were rotated by 90° to make seedlings horizontal. The images were recorded every 10 min for 6 h, or 12 h or 24 h (depending upon the experiment) using a Quickcam Pro 3000. Each petriplate contained 4-6 seedlings, and the experiment was repeated about 4 times (Figure 5D).
- The gravitropic response of tomato seedlings was examined under omnilateral blue light (white light can also be used). To eliminate any phototropic effect of light on seedlings the time lapse imaging can be done using Infra-red LEDs as a light source. Figure 4 shows the placement of camera and seedlings. Seedlings were placed at a horizontal distance of 20 cm from the camera lens. The light source was 20 cm above the seedlings.
- Light sources
In the current experiment we used omnilateral low fluence blue light (λmax 470 nm) obtained using blue LEDs to capture the movement of the seedlings with respect to gravity. The experiment can also be done in infrared light for eliminating any likely effect of plant photoreceptors i. e. under complete darkness. Unless otherwise mentioned, “low-fluence blue light” (3 µmol m-2 s-1) was used to provide minimum light to camera to capture the images. - Time lapse video imaging and analysis
- Time lapse images were captured using Quickcam-Pro 4000 (see Whippo and Hangarter, 2003) attached to the computer.
- For creating a time lapse video image (Video 1), frames were extracted at specific time points using either MGI VideoWave4 PC video editing (USA) or Time lapse imaging software from the movie. Images were captured at 10 min interval after gravistimulation or after orienting plate by 90°. The time lapse frames were fused to create a video using the above software.
- The background illumination was provided by using low fluence blue light LEDs. It can be replaced by infrared LEDs (λmax 940 nm).
- The movie shows the negative gravitropic response of hypocotyls of tomato seedlings after the seedlings were horizontally oriented. After a lag period (~20 min), gravitropism resulted in differential growth of hypocotyls leading to bending of seedlings away from gravity vector. Within an hour, the hypocotyls reorient to vertical growth, and regain the same growth orientation that was before onset of gravistimulation.
- Angle measurements
The curvature angles were calculated after subtracting the zero point images from the images obtained at defined time points. All measurements were performed using NIH ImageJ software and e-Ruler r. The graph was plotted using SigmaPlot 10.0. Figure 6 shows snapshot of angle measurement using the ImageJ software. - Figure 7 shows the time course of gravitropic curvature of two day-old etiolated seedlings.
- Time lapse images were captured using Quickcam-Pro 4000 (see Whippo and Hangarter, 2003) attached to the computer.
Representative data
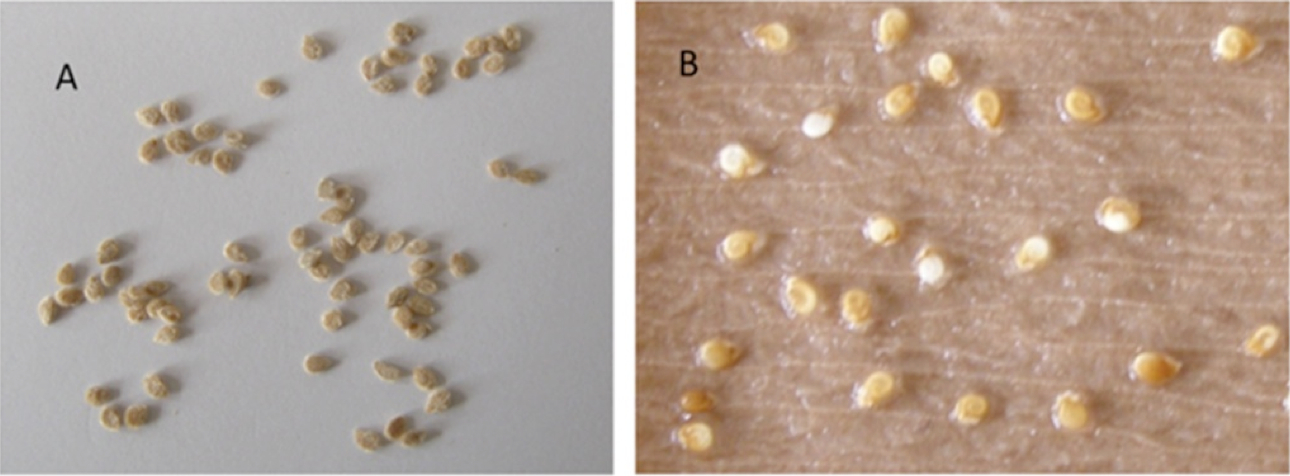
Figure 1. A. Seeds were selected for the experiment. B. The seeds after treatment with 20% (v/v) sodium hypochlorite for 15 min. The treatment with sodium hypochlorite leads to thinning of seed coat and coiled embryo like structure is visible leading to uniform germination of the seeds. 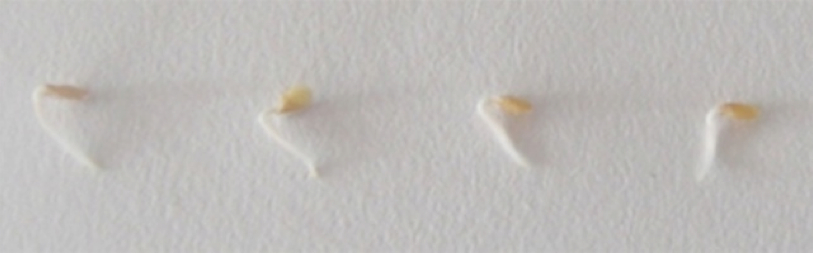
Figure 2. 3-days-old etiolated germinated seeds of tomato. After emergence of radicle from seed, the seeds were transferred on to 0.8% (w/v) agar. 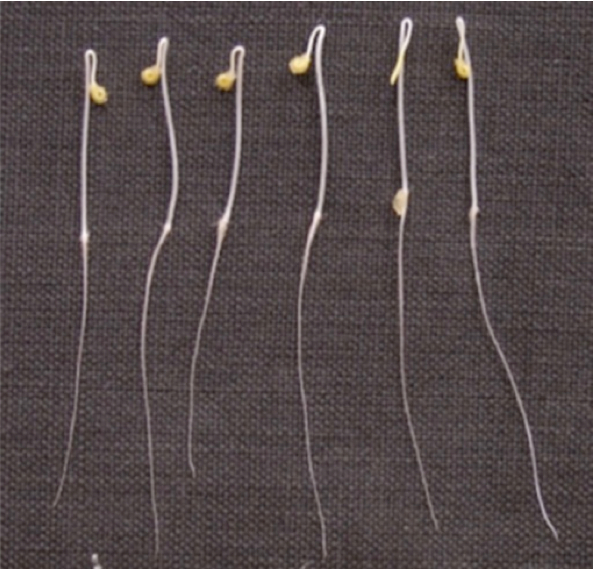
Figure 3. Two day-old etiolated seedlings of tomato. Seedlings of same height were used for the analysis.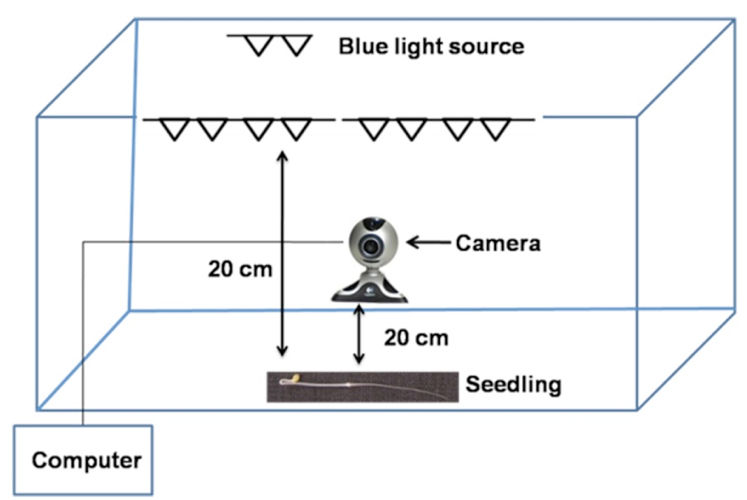
Figure 4. Graphical illustration of time-lapse recording setup used for analyzing gravitropic response. The entire set-up was enclosed in wooden chamber placed in a dark room. The images were recorded at defined interval by a computer connected to the camera. Computer was placed outside the wooden chamber and the computer screen was either switched off or covered with a black cloth during the entire duration of recording.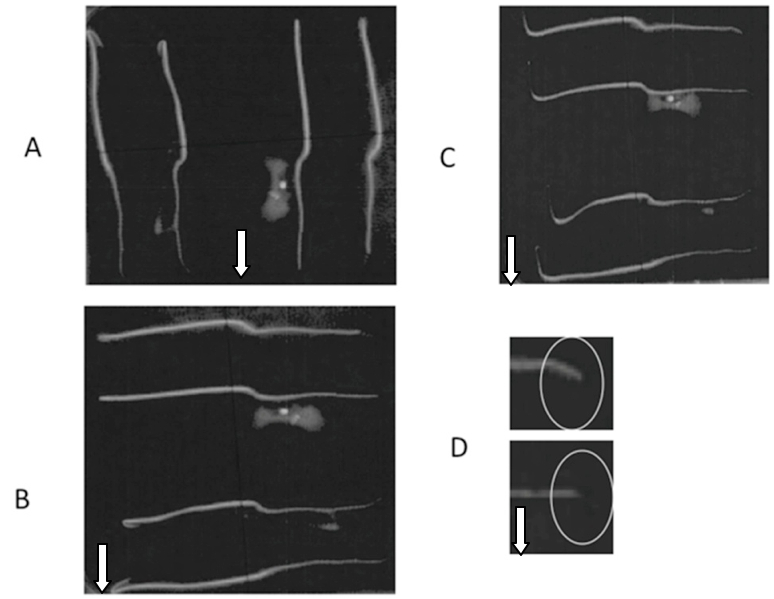
Figure 5. A. The seeds after emergence of radicle were grown on 0.8% (w/v) agar in a petriplate. B. After two days, the petriplate was rotated at 90° for gravistimulation. C. Negative gravitropism of hypocotyl after 2 h. D. Positive gravitropism of root tip towards gravity. The white arrow in picture indicates the direction of gravitational field.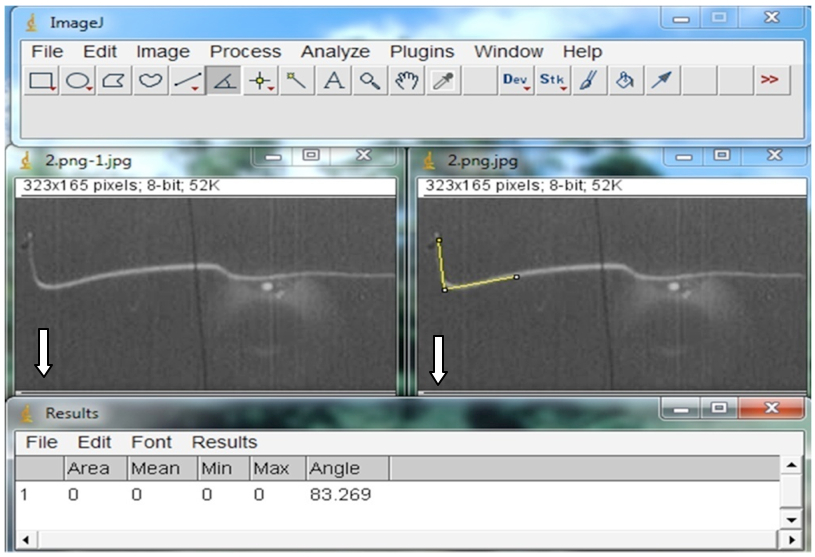
Figure 6. The yellow lines in right snapshot shows the measurement of hypocotyl angle by ImageJ software. The white arrow in picture indicates the direction of gravitational field.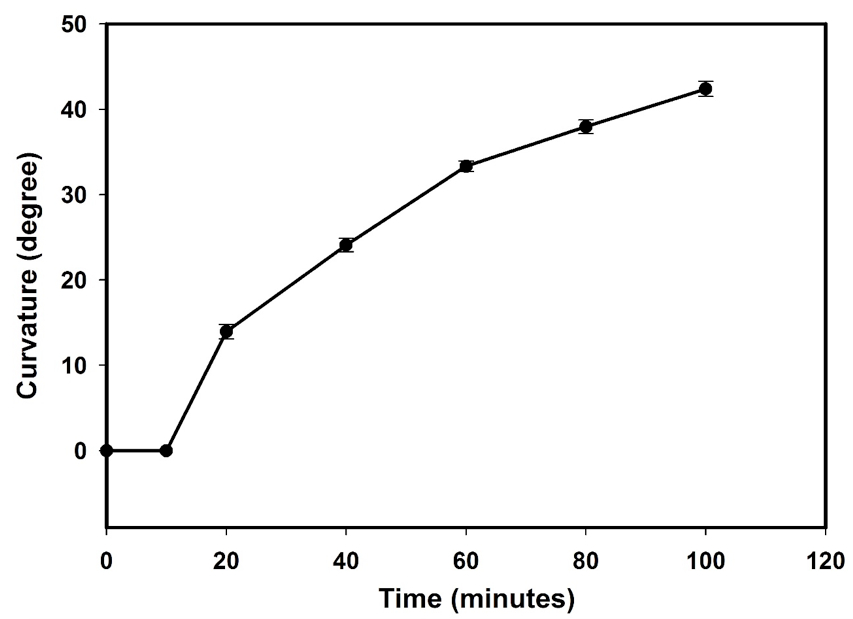
Figure 7. Time course of hypocotyl gravitropic curvature in two-day old etiolated tomato seedlings. The curvature angles of individual hypocotyls were measured at defined time intervals for 120 min by time lapse photography. The data points represent the mean value ± S.E of 10 individual seedlings from three independent experiments.
Notes
- The age of the seedlings is an important factor for the reproducibility of the experiment. The seedlings should be of uniform length to reduce the error. Tomato seedlings taller than 3.5 cm show poor hypocotyl tropism.
- The treatment with sodium hypochlorite should be carefully monitored as efficacy of sodium hypochlorite is variable and depends on batch and company used. The longer hypochlorite treatment or stronger hypochlorite solution treatment can kill the seeds.
Recipes
- 0.8% (w/v) agar
Weigh 0.8 gm agar
Add 100 ml double distilled water
Boil for 2-3 minutes in a beaker
Cool down and pour into the square petriplate
Acknowledgments
Financial support provided by the Council of Scientific and Industrial Research (CSIR), New Delhi, in the form of SRF to Kamal Tyagi is gratefully acknowledged. This work was supported by Department of Biotechnology, New Delhi grant to RS and YS.
References
- Whippo, C. W. and Hangarter, R. P. (2003). Second positive phototropism results from coordinated co-action of the phototropins and cryptochromes. Plant Physiol 132(3): 1499-1507.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sharma, S., Tyagi, K., Sreelakshmi, Y. and Sharma, R. (2015). Gravitropic Analysis of Tomato Seedlings using Time Lapse Video Imaging. Bio-protocol 5(7): e1443. DOI: 10.21769/BioProtoc.1443.
- Whippo, C. W. and Hangarter, R. P. (2003). Second positive phototropism results from coordinated co-action of the phototropins and cryptochromes. Plant Physiol 132(3): 1499-1507.
Category
Plant Science > Plant physiology > Plant growth
Plant Science > Plant developmental biology > Gravitropism
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










