- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cyst Detection in Toxoplasma gondii Infected Mice and Rats Brain
Published: Vol 5, Iss 7, Apr 5, 2015 DOI: 10.21769/BioProtoc.1439 Views: 10529
Reviewed by: Fanglian HeAlexandros Alexandratos

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Triple-challenge Mouse Model of Allergic Airway Disease, Primary Influenza Infection, and Secondary Bacterial Infection
Sean Roberts [...] Yoichi Furuya
Apr 20, 2020 4533 Views

RNA Extraction from Ears and Draining Lymph Nodes of Mice Infected with Leishmania amazonensis
Emilie Giraud and Evie Melanitou
Jun 5, 2020 5517 Views

TetR Regulated in vivo Repression Technology to Identify Conditional Gene Silencing in Genetically Engineerable Bacteria Using Vibrio cholerae Murine Infections as Model System
Franz G. Zingl [...] Stefan Schild
Oct 5, 2020 3750 Views
Abstract
Toxoplasmosis caused by the intracellular parasite Toxoplasma gondii, is characterized by a life-long chronic infection. The parasite is an efficient neurotropic infectious agent that establishes its “safe” life by forming intracellular cysts in chronically infected animals and humans. This protocol describes the specific recipes and method to stain brain cysts from infected mice and rats for further quantification using epifluorescence microscopy. This method provides the possibility to scan the entire brain and thus to numerate all cysts.
Keywords: Toxoplasma gondiiMaterials and Reagents
- Human foreskin fibroblasts (HFFs) cells culture (ATTC, catalog number: SCRC-1042 )
- Dulbecco's modified Eagle medium (DMEM) (Life Technologies, catalog number: 41966-029/052 )
- Fetal bovine serum (FBS) (Eurobio®, catalog number: CVFSVF0001 )
- Penicillin/streptomycin (PAN Biotech GmbH, catalog number: P0607-100 )
- L-Glutamine (200 mM) (Life Technologies, catalog number: 25030-024 )
- Dulbecco's phosphate-buffered saline (DPBS) (Life Technologies, catalog number: 14190-094/069 )
- Proteinase K (molecular biology grade) (Bio-Rad Laboratories, catalog number: P8107S , Lot: 0051310)
- TRIS (Euromedex, catalog number: 26-128-3094-B )
- EDTA (Euromedex, catalog number: E013 )
- SDS (Euromedex, catalog number: EU0660 )
- NaCl (Euromedex, catalog number: 1112-A )
- HCl (37% ACS reagent) (Sigma-Aldrich, catalog number: 258148-2.5ML )
- Phenylmethylsulfonylfluoride (PMSF) (Euromedex, catalog number: 1111-C )
- Hoescht 33258 (Life Technologies, Molecular Probes®, catalog number: H-3569 )
- Formaldehyde methanol free (Polysciences, catalog number: 0 4018 )
- Dolichos biflorus lectin coupled to fluorescein isothiocyanate (FITC) (Clinisciences, catalog number: FL-1031 )
- Complete DMEM medium (see Recipes)
- Phenylmethylsulfonylfluoride (PMSF) solution (see Recipes)
- Lysis buffer (see Recipes)
Equipment
- 37 °C/5% CO2 cell culture incubator
- 6-well plates
- Scissors and forceps
- Glass homogenizers (Potter-Elvehjem PTFE, 15 ml) (Dutscher, catalog numbers: 057009 and 057021 )
- 15 ml polystyrene tubes
- Epifluorescence microscope
Procedure
- Preparation of Human Foreskin Fibroblasts cell culture in 6-well plates
Plate HFFs cells in complete DMEM medium and culture them for 4 days at 37 °C in presence of CO2. HFFs have to be at 100% confluence in each well (8 x 105 cells) before staining.
- Staining of HFFs
- Wash twice the cells culture with 1 ml 1x DPBS.
- Fix cells with 1 ml formaldehyde diluted at 2.5% in DPBS 1x for 20 min at 4 °C.
- Incubate 20 min, in the dark and at RT, the cells with 1 ml of Hoescht diluted 1/50,000 in 1x DPBS.
- Wash twice the cells culture with 1 ml 1x DPBS.
- Add 1 ml 1x DPBS.
- Stored at 4 °C until addition of the stained homogenized brain.
Note: The stained cells are stored at 4 °C for a maximum of 24 h.
- Wash twice the cells culture with 1 ml 1x DPBS.
- Isolation of brains from infected mice or rats
- Anesthesize animal with isofluoran and euthanize it by cervical dislocation.
- Soak the head with 70% (v/v) ethanol.
- Cut the skin at the base of skull and remove it as much as possible.
- Cut with scissors the dorsal and lateral part of the skull and take the top off.
- Collect the brain tissue in 5 ml DPBS 1x solution.
- Anesthesize animal with isofluoran and euthanize it by cervical dislocation.
- Brains homogenization
Put each mouse brain in 2 ml DPBS 1x into a glass homogenizer, homogenize at RT and adjust final volume to 4 ml. For rat brain, homogenize in 4 ml and adjust to 8 ml final.
Note: Brains can be stored at 4 °C until the staining for no more than 2 days.
- Brains staining
- Prepare 5x lysis buffer and proteinase K at 8 U/ml (stock at 800 U/ml, 1/100 dilution in 5x lysis buffer just before use).
- For mice, take 1 ml of homogenized brain (¼ brain), add 398 µl of 5x lysis buffer, 2 µl of proteinase K (see step E1, so final concentration at 0.008 U/ml) and 600 µl of 1x DPBS (final volume of 2 ml).
For rats, take 2 ml of homogenized brain (¼ brain), add 995 µl of 5x lysis buffer, 5 µl of proteinase K (see step E1, so final concentration at 0.008 U/ml) and 2 ml of 1x DPBS (final volume of 5 ml).
- Incubate at 56 °C for 15 min, homogenize every 5 min by vortexing.
- Stop the proteinase K activity by adding PMSF to a final concentration of 2 mM (stock at 200 mM), homogenize and incubate at RT for 5 min.
- Centrifuge the sample for 15 min at 1,250 x g (RT).
- Gently eliminate the supernatant by pipetting.
- Resuspend in 1 ml of Dolichos biflorus lectin diluted at 1/250 in DPBS 1x (996 µl 1x DPBS + 4 µl of Dolichos biflorus lectin).
- Incubate 30 min at RT in a mechanical wheel placed in the dark.
- Add 3 ml DPBS 1x and homogenize to wash the sample.
- Centrifuge 15 min at 1,250 x g (RT).
- Gently eliminate the supernatant by pipetting.
- Resuspend each sample in 1 ml in DPBS 1x and transfer onto Hoescht-stained HFFs (see part A and B of the procedure) by pipetting (1 ml/well).
Note: It is important to transfer the homogenized brain pellet onto HFF cells to facilitate microscope focus when there are no or very few cysts.
- Prepare 5x lysis buffer and proteinase K at 8 U/ml (stock at 800 U/ml, 1/100 dilution in 5x lysis buffer just before use).
- Count the cysts using epifluorescence microscopy (Representative data)
Note: Samples can be stored at 4 °C until microscope observation for no more than 10 days. To know the cysts quantity in entire brain, multiply the counted number by 4.
Representative data
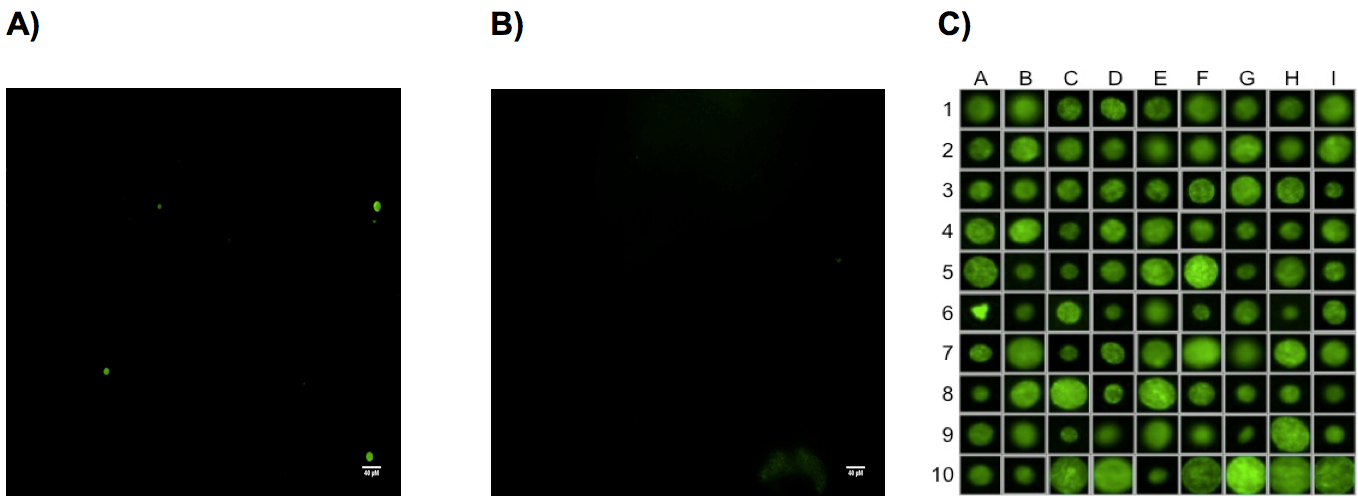
Figure 1. Representative cysts staining from uninfected and infected CBA mice brain with Toxoplasma gondii parasites. Two months after i.p. infection of CBA mice with 1,000 ΔKu80-ΔHXGPRT (PRU) tachyzoïtes, brains were collected, homogenized in 4 ml of PBS and ¼ of each brain suspension was used for the labeling of cyst walls with the D. biflorus-FITC lectin and analyzed by high content screening microscopy (Scan^R Olympus). A) Photo (4x objective) of a CBA infected mouse brain. Three stained cysts are present in this field. B) Uninfected CBA mouse brain (4x objective) no cysts are visible but only brain debris. C) Representative panel of stained cysts obtained from infected CBA mouse and detected by (4x objective) microscopy. Cysts are zoomed to detect potential false positive as in A6.
Recipes
- Complete DMEM medium
10% (v/v) FBS
0.5% (v/v) penicillin/streptomycin
2 mM glutamine
- Phenylmethylsulfonylfluoride (PMSF) solution (stock concentration of 200 mM)
0.35 g of PMSF powder to dissolve in 10 ml of isopropanol
Stored at -20 °C in 1 ml aliquots
Note: PMSF will take some time to dissolve.
- Lysis buffer (500 ml)
10M TRIS: 2.5 ml of 2 M TRIS at pH 8.0 (60 g in 250 ml H2O, adjust pH)
1 mM EDTA: 2 ml of 250 mM EDTA at pH 8.0 (23.27 g in 250 ml H2O, adjust pH)
0.2% (w/v) SDS: 5 ml of 20% (w/v) SDS
100 mM NaCl (1.165 g)
Up to 500 ml H2O
Note: The 8.0 pH is adjusted using HCI 3 M (HCl 3 M: 25 ml HCl 37% added to 75 ml of H2O).
Acknowledgments
This work was supported by the ANR grants Nu 05-MIIM-020-02 and Nu 07-MIME-013-01, the Lyonbiopole competitiveness cluster and the French Parasitology consortium ParaFrap (ANR-11-LABX0024).
References
- Aldebert, D., Hypolite, M., Cavailles, P., Touquet, B., Flori, P., Loeuillet, C. and Cesbron-Delauw, M. F. (2011). Development of high-throughput methods to quantify cysts of Toxoplasma gondii. Cytometry A 79(11): 952-958.
- Cavailles, P., Flori, P., Papapietro, O., Bisanz, C., Lagrange, D., Pilloux, L., Massera, C., Cristinelli, S., Jublot, D., Bastien, O., Loeuillet, C., Aldebert, D., Touquet, B., Fournie, G. J. and Cesbron-Delauw, M. F. (2014). A highly conserved Toxo1 haplotype directs resistance to toxoplasmosis and its associated caspase-1 dependent killing of parasite and host macrophage. PLoS Pathog 10(4): e1004005.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bellini, V., Loeuillet, C., Massera, C., Cesbron-Delauw, M. and Cavaillès, P. (2015). Cyst Detection in Toxoplasma gondii Infected Mice and Rats Brain. Bio-protocol 5(7): e1439. DOI: 10.21769/BioProtoc.1439.
Category
Microbiology > Microbe-host interactions > In vivo model > Mammal
Cell Biology > Cell imaging > Fluorescence
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link