- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Arabidopsis thaliana Root Hair Cell Cytoplasmic pH (pHc) Imaging
Published: Vol 5, Iss 7, Apr 5, 2015 DOI: 10.21769/BioProtoc.1438 Views: 9834

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions
Yuta Kato [...] Mitsutaka Taniguchi
Aug 5, 2025 2323 Views

Live-Cell Monitoring of Piecemeal Chloroplast Autophagy
Masanori Izumi [...] Shinya Hagihara
Nov 5, 2025 1691 Views

Chloroplast Movement Imaging Under Different Light Regimes With a Hyperspectral Camera
Paweł Hermanowicz [...] Justyna Łabuz
Dec 20, 2025 758 Views
Abstract
For monitoring the cellular pH in plants, engineered green fluorescent protein (GFP) has been used to indicate cellular pH. Pt-GFP is a pH-reporter protein which has been used ratiometrically in the double excitation mode, according to the fluorescence properties described for Pt-GFP (Schulte et al., 2006), and depending on our previous experiments, we present here a little and efficient protocol for monitoring the cytosolic pH value. In this protocol, root hair cellular pH was monitored in the PtGFP reporter lines. Cellular pH can be obtained according to the calibration curve which was performed in situ by using a series pH buffer.
Keywords: Cytoplasmic pHMaterials and Reagents
- Stable Pt-GFP transgenic WT line N9561 (background: Col-0) was obtained from the Nottingham Arabidopsis Stock Centre http://arabidopsis.info/StockInfo?NASC_id=9561).
Note: Pt-GFP T-DNA insertion mutant homozygous were obtained by crossing the T-DNA insertion mutant (what you interested, here meant the mutant with root hair growth problem) with the N9561. And we obtained the T-DNA insertion homozygous first by PCR (methods in http://signal.salk.edu/cgi-bin/tdnaexpress) in the F2 progeny, and we checked the fluorescence for Pt-GFP. Homozygous lines with fluorescence will be used for later analysis.
- Digitonin (Sigma-Aldrich, catalog number: 11024-24-1 )
Note: Risk assessment: toxic. Digitonin is toxic by inhalation, contact with skin, wear protective gloves and clothing, wear respiratory protection, when handling this chemical.
- 2-(N-Morpholino) ethanesulfonic acid (MES) (Sigma-Aldrich, catalog number: 145224-94-8 )
- N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: 7365-45-9 )
- Murashige and Skoog (MS) medium (Phyto Tech, catalog number: M519 )
- pH buffer solutions (see Recipes)
Equipment
- Inverted Carl Zeiss LSM 710 confocal microscope
- Plant growth chamber
- 2 ml tubes (Eppendorf tubes)
- Forceps
- Microscope slides
- Microscope cover slips
Software
- Microsoft Excel
Procedure
- Surface-sterilized Arabidopsis seeds were sowed on 0.5 x MS medium and kept for 3 d at 4 °C in the dark. The plates were then transferred to the growth chamber at 22 °C with a 16-h light/8-h dark photoperiod and grown vertically. Pt–GFP Root hairs of 4-5-d-old seedlings were used to monitor intracellular pH. Seedlings were carefully placed on the slide with 2-3 drops of liquid growth medium (the same as the medium seedling were grown on). Root hair GFP fluorescence was monitored under the confocal microscope (exciters: 405 nm and 488 nm; emitter: 525 nm); the F405-to-F488 ratio was used as a measure for pH, and a pseudocolor-coded 405 nm/488 nm ratio image can be obtained.
- The pH titrations were performed in situ as previously reported (Moseyko and Feldman, 2001). For making the calibration curve, a series of pH buffer solutions (5, 5.5, 6, 6.5, 7, 7.5 and 8; for preparation, see Recipes) were used, 4-5-d-old seedlings were incubated in the buffer solution and were stabilized for about 10 min to equilibrate the external pH buffer solutions and the pHc. Fluorescence was made as procedure 1 to obtain F405-to-F488 ratio for different pH buffer solution. For each, at least 5 repeats should be made.
- After the in situ titration, an equation (R2 near 0.999) will be reached for conversion the fluorescence ratio to the pH value by using the Microsoft Excel. pH levels of the root hair obtained in step 1 were then calibrated by the equation.
- Waste disposal
Offer surplus and non-recyclable solutions with digitonin to a licensed disposal company. Contact a licensed professional waste disposal service to dispose of this material.
Representative data
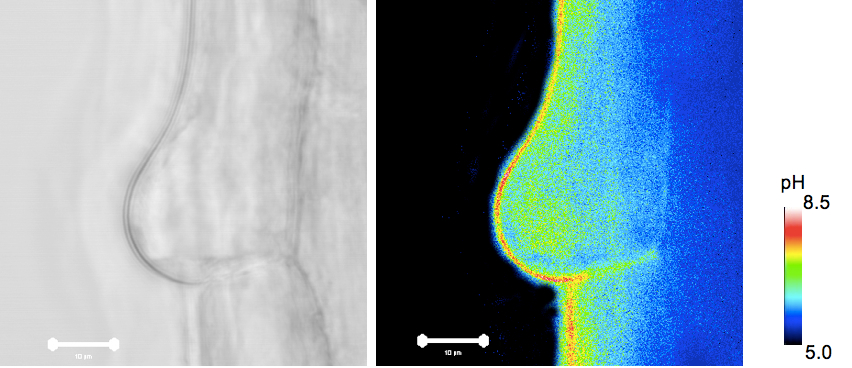
Figure 1. pHc in root hairs of Pt-GFP plants. Bars = 10 µm
Recipes
- pH buffer solutions
0.5 x Murashige and Skoog basal salt mix (MS) salts
0.005% digitonin
50 mM MES/NaOH (for adjusting pH to 5, 5.5, 6, 6.5) or 20 mM HEPES/NaOH (for pH to 7, 7.5, 8)
Acknowledgments
This work was supported by the National Key Basic Special Funds (2012CB1143001).
References
- Moseyko, N. and Feldman, L. J. (2001). Expression of pH-sensitive green fluorescent protein in Arabidopsis thaliana. Plant Cell Environ 24(5): 557-563.
- Schulte, A., Bottcher, M., Lorenzen, I. and Plieth, C. (2006). A novel pH indicator for expression in plants. Plant Methods 2:7.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bai, L., Zhou, Y., Wang, P. and Song, C. (2015). Arabidopsis thaliana Root Hair Cell Cytoplasmic pH (pHc) Imaging . Bio-protocol 5(7): e1438. DOI: 10.21769/BioProtoc.1438.
Category
Plant Science > Plant physiology > Ion analysis
Plant Science > Plant cell biology > Cell imaging
Cell Biology > Cell imaging > Fluorescence
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









