- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Staining of Callose Depositions in Root and Leaf Tissues
Published: Vol 5, Iss 6, Mar 20, 2015 DOI: 10.21769/BioProtoc.1429 Views: 27828
Reviewed by: Ru ZhangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1897 Views

New Approach to Detect and Isolate Rhamnogalacturonan-II in Arabidopsis thaliana Seed Mucilage
Dayan Sanhueza and Susana Saez-Aguayo
Sep 5, 2025 1248 Views

Detailed Method for the Purification of Rhamnogalacturonan-I (RG-I) in Arabidopsis thaliana
Liang Zhang [...] Breeanna R. Urbanowicz
Feb 5, 2026 144 Views
Abstract
The plant cell wall is a physical barrier, which fulfills a plethora of functions, for example it can efficiently prevent pathogen’s entry into the cell. In addition, its changing composition contributes to plants inducible defense mechanisms. This layer of defense includes pathogen perception and is followed by the activation of defense responses resulting, among others, in a modification and remodeling of the cell wall. This relatively late defense response (hours or days after contact with pathogen) comprises the accumulation of polysaccharides, such as the 1,3-ß-glucan callose, phenolic compounds and reactive oxygen species. Callose depositions occur during normal plant growth (e.g. in the phloem), they can be also a response to different stress stimuli. During the response to pathogen attack, callose depositions are essential part of cell wall reinforcement and are important for successful plant defense. Here, we describe a method to stain callose apposition spots, which can be used to quantify this defense response.
Materials and Reagents
- Plant material
- Flg22 (a flagellin-derived, 22 amino acid-long peptide) (QRLSTGSRINSAKDDAAGLQIA)
- 100% ethanol
- 100% acetic acid (Carl Roth, catalog number: 3738.5 )
- K2HPO4 (Carl Roth, catalog number: P749.2 )
- Aniline blue or water blue (Fluka Chemika, catalog number: 95290)
- Glycerol (Carl Roth, catalog number: 7530.4 )
- Filter paper (Munktell, catalog number: 3.00343.080)
- Destaining solution (see Recipes)
- Wash solution (see Recipes)
- Staining solution (see Recipes)
Equipment
- Plastic equipment allowing to float plant material in staining solution (Grainer Falcon 50 ml tubes, catalog number: 227261 ; Grainer 6-well culture plates, catalog number: 657160 )
- Vacuum pump (Savant Systems LLC, catalog number: SC110 : 25-30 Hg)
- Microscope (Zeiss, model: Axioplan 2ie) with UV lamp (FluoArc/001.26B) and ‘DAPI’ filter (excitation filter 390 nm; dichroic mirror 420 nm; emission filter 460 nm)
Procedure
- Plant material:
We used either detached leaves from Arabidopsis plants or seedlings, depending on the experimental setup and the overall aim of the experiment. Plants and seedlings were grown in two different systems:
- Arabidopsis plants were grown on soil under short day conditions (8/16 h day/night photoperiod) at 21 °C for 5 weeks. Before the elicitor challenge, detached leaves were floated on plant growth media (½ MS) for 3 days for the appropriate pretreatment (Figure 1A).
- Arabidopsis seeds were surface-disinfected, germinated, and grown for 2 weeks on ½ MS plant growth media. Thereafter, seedlings were transferred into liquid plant growth media in 6-well plates for 3 days for the appropriate pretreatment (Figure 1B). The growth conditions were set to 22 °C with 150 µmol/m2/s light in 8/16 h day/night photoperiod and 65 % relative humidity.
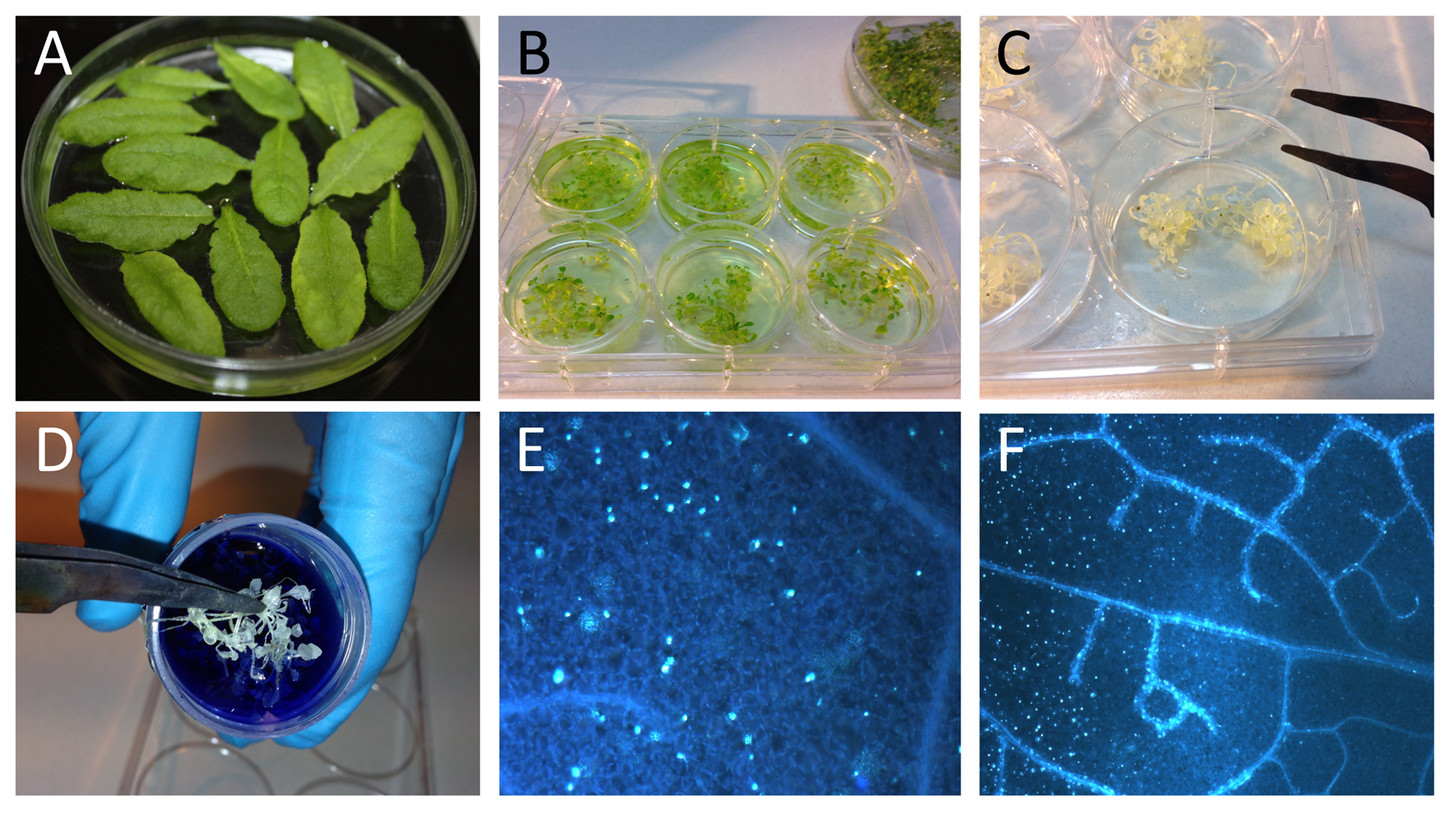
Figure 1. Plant material, staining with aniline blue and destaining. A. Leaves detached from 5-week-old, soil-grown Arabidopsis plants were floated on plant growth medium. B. Two-week-old Arabidopsis seedlings were floated on plant growth medium in 6-well plates. C. Translucent Arabidopsis seedlings after destaining. D. Arabidopsis seedlings transferred into aniline blue staining solution. E-F. Microscopic visualization of callose depositions.
- Arabidopsis plants were grown on soil under short day conditions (8/16 h day/night photoperiod) at 21 °C for 5 weeks. Before the elicitor challenge, detached leaves were floated on plant growth media (½ MS) for 3 days for the appropriate pretreatment (Figure 1A).
- Plants were challenged with flagellin (flg22) to activate their defense responses. In the case of seedlings, flg22 was added to the medium at a final concentration of 100 nM for 24 h. For detached leaves, 1 µM flg22 solution was sprayed and then briefly vacuum infiltrated (Petri dishes with floating leaves were placed in a closed box until a pressure reached 0.84 bar), leaves were incubated on wet filter paper for 24 h.
- After 24 h incubation, plants or leaves were fixated and destained in 1:3 acetic acid/ethanol until the material was transparent (usually over night), the saturated destaining solution was replaced, if necessary (Figure 1C).
- Fixated and destained leaves or seedlings were washed in 150 mM K2HPO4 for 30 min.
- Plant material was incubated for at least 2 h in 150 mM K2HPO4 and 0.01% aniline blue (staining solution) in a Falcon tube wrapped in aluminium foil for light protection (Figure 1D).
- In order to facilitate the observations samples were embedded in 50% glycerol before further analysis. The embedment in 50% glycerol allows a prolonged observation time and reduces the disturbances caused by air bubbles.
- Callose depositions were quantified in a fluorescence microscope using a DAPI filter (Figure 1E-F). The optimal excitation wavelength for aniline blue is 370 nm, and the emission maximum is by 509 nm. For documentation we used the Zeiss AxioVision 3.0 software. The images were acquired with 1,300 x 1,030 resolution and left untreated except for the adjustment of brightness. Generally we used 300 ms exposure time.
Representative data
- For representative data see Schenk et al. (2014).
- For reproducibility see Notes.
Notes
Callose depositions can be easily quantified by counting all stained spots in the defined leaf area(s). In addition, the amount of deposits can be categorized in groups, ranked from the lowest to the highest density of spots per leaf or leaf area. Quantification of the intensity of callose with image analysis software is also possible, however focusing of the image to visualize all callose depositions in a particular leaf area may be difficult. To achieve high reproducibility of results, it is important to perform the experiments with leaves at equal age and to prevent injuries during the transfer of plants or leaves to the different solutions during sample preparation.
Recipes
- Destaining solution
1:3 acetic acid/ethanol
- Wash solution
5.2 g of K2HPO4 in 200 ml H2O
- Staining solution
1.3 g of K2HPO4 with 5 mg of aniline blue in 50 ml H2O
Acknowledgments
The protocol was modified after Clay et al. (2009). This work was supported by the Federal Office for Agriculture and Food (BLE), Germany.
References
- Clay, N. K., Adio, A. M., Denoux, C., Jander, G. and Ausubel, F. M. (2009). Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323(5910): 95-101.
- Schenk, S. T., Hernandez-Reyes, C., Samans, B., Stein, E., Neumann, C., Schikora, M., Reichelt, M., Mithofer, A., Becker, A., Kogel, K. H. and Schikora, A. (2014). N-Acyl-Homoserine lactone primes plants for cell wall reinforcement and induces resistance to bacterial pathogens via the salicylic acid/oxylipin pathway. Plant Cell 26(6): 2708-2723.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Schenk, S. T. and Schikora, A. (2015). Staining of Callose Depositions in Root and Leaf Tissues. Bio-protocol 5(6): e1429. DOI: 10.21769/BioProtoc.1429.
Category
Plant Science > Plant biochemistry > Carbohydrate
Plant Science > Plant cell biology > Cell staining
Plant Science > Plant immunity > Disease bioassay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









