- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Vesicle Isolation from Bacillus subtilis Biofilm
Published: Vol 5, Iss 5, Mar 5, 2015 DOI: 10.21769/BioProtoc.1409 Views: 13375
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Extraction of Bacterial Membrane Vesicle and Phage Complex by Density Gradient Ultracentrifugation
Shangru Li [...] Tianyuan Jia
Aug 20, 2024 2054 Views

Mycobacterium smegmatis Ribosome Purification, Co-sedimentation, and Subunit Association Assay
Aneek Banerjee [...] Jayati Sengupta
May 20, 2025 1580 Views

Preparation and Negative Staining for Visualization of Cyanoglobule Lipid Droplets Using Transmission Electron Microscopy
Febri A. Susanto [...] Peter K. Lundquist
Dec 5, 2025 1311 Views
Abstract
Bacterial biofilms are associated clinically with many bacterial infections including those caused by bacteria such as Pseudomonas aeruginosa and Staphylococcus aureus. In recent years, extracellular vesicles produced by bacteria have been isolated from biofilm communities. Vesicles have been described in depth and can encapsulate various virulence factors including toxins and immunomodulatory compounds. Vesicles may be important for virulence and survival by serving as a vehicle for the secretion and concentrated delivery of these molecules. Studying extracellular vesicles is an important step towards understanding biofilm formation, structure, and disruption with the ultimate goal of preventing or treating hospital infections caused by bacterial pathogens residing in biofilms. Here we describe the protocol for isolating vesicles from biofilm produced by Bacillus subtilis.
Materials and Reagents
- Bacillus subtilis (B. subtilis) 168 bacterial strain (available from ATCC, catalog number: 23857 )
- BHI broth/agar (BD, catalog number: 211059/211065)
- Amicon Ultra centrifugal filter units Ultra-4 (MWCO 100 kDa) (Millipore, catalog number: UFC910024 )
- 1x phosphate buffered saline (PBS) (see Recipes)
- MSgg medium (see Recipes)
Equipment
- Petri dishes (100 x 55 mm) (Corning, catalog number: 351029 ) (dish size can be variable and ultimately depends on the amount of vesicles you wish to purify)
- 0.22 μm syringe filters (Fisher, catalog number: 09-719C)
- Tube (thickwall, polyallomer, 3.5 ml, 13 x 51 mm) (Beckman Coulter, catalog number: 349623 )
- Optima TL ultracentrifuge (Beckman Coulter)
- TLA 100.3 rotor (Beckman Coulter)
- Centrifuge capable of 15,000 x g
Procedure
- Biofilm growth assay
- Inoculate a plate of Bacillus subtilis lab strain 168 (from -80 °C) on a BHI agar plate overnight (~18 h) at 37 °C.
- Inoculate 5 ml BHI broth from overnight agar plate (one colony) and grow for 4 h with shaking (~200 rpm) at 37 °C.
- Inoculate 1:1,000 of broth culture (12 μl) into 12 ml MSgg media in petri dishes.
- Let biofilm incubate (covered with dish lid without shaking) at 37 °C for desired time period (usually 3 and 7 days). Ensure that incubator has a water container to ensure the dishes do not dry out.
- Inoculate a plate of Bacillus subtilis lab strain 168 (from -80 °C) on a BHI agar plate overnight (~18 h) at 37 °C.
- Purification of vesicles from biofilm
- After desired time period, remove pellicle and supernatant from plates by pipetting and centrifuge at 15,000 x g for 20 min at 4 °C to remove cells and large debris.
- To remove remaining cells and debris, filter the spun supernatant with 0.22 μm syringe filters.
- Centrifuge filtered, cell-free supernatant with centrifugal filter units to concentrate to less than 3 ml in volume.
- To pellet vesicles from supernatant, ultracentrifuge concentrated supernatant at 195,000 x g for 1 h at 4 °C in polyallomer tubes.
- Remove supernatant without disturbing the vesicle pellet by pipetting.
- Repeat spin after washing in 500 μl PBS.
- Remove PBS wash from tube without disturbing the vesicle pellet.
- Resuspend pellet in desired volume of PBS.
- After desired time period, remove pellicle and supernatant from plates by pipetting and centrifuge at 15,000 x g for 20 min at 4 °C to remove cells and large debris.
Notes
- Bacillus subtilis grows as a biofilm pellicle on the surface of the media (Figure 1). It may need a few washings to remove all cells for vesicle collection. Figure 2 shows electron micrograph of negatively stained vesicles.
- Biofilms can be grown in various plates to achieve desired volume of vesicles. Ideally you want to have a visible pellet after ultracentrifugation. If no pellet is visible, scale up the plate size or number of biofilms for vesicle purification.
- Vesicle pellet after ultracentrifugation should be visible, but in cases of small culture volumes the pellet may be difficult to see.

Figure 1. Representation of what biofilm pellicles may look like in a 6-well plate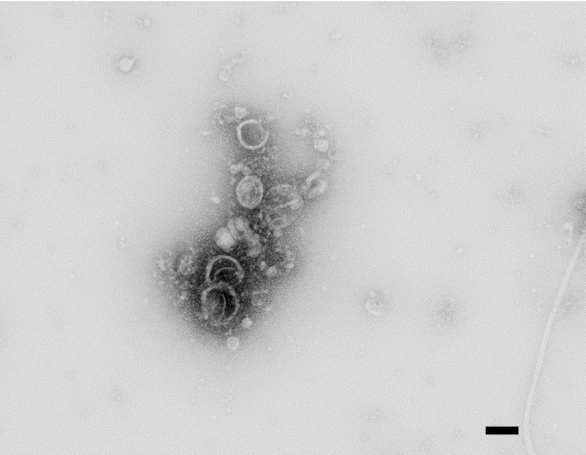
Figure 2. Negative stain TEM of vesicles isolated from Bacillus subtilis biofilm. Scale bar=100 nm
Recipes
- 1x phosphate buffered saline (PBS) (1 L, pH 7.4) (filter or autoclave sterilize)
137 mM NaCl
2.7 mM KCl
10 mM Na2HPO4
1.8 mM KH2PO4
Bring to pH 7.4 with HCl
Dissolve in H2O up to 1 L - MSgg medium (pH 7) (filter sterilize)
50 μM MnCl2
5 mM KH2PO4
1 μM ZnCl2
50 μM FeCl3
2 mM MgCl2
700 μM CaCl2
50 μg/ml threonine, tryptophan, and phenylalanine
0.5% glutamate
0.5% glycerol
2 μM thiamine
100 mM morpholinepropanesulfonic acid (MOPS) (pH 7)
Acknowledgments
Funding from NIH Grant Numbers: HL059842, AI033774, AI033142, AI052733 and Center for AIDS Research at Albert Einstein College of Medicine.
Protocol was adapted from Brown et al. (2014) and McLoon et al. (2011).
References
- Brown, L., Kessler, A., Cabezas-Sanchez, P., Luque-Garcia, J. L. and Casadevall, A. (2014). Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Mol Microbiol 93(1): 183-198.
- Prados-Rosales, R., Brown, L., Casadevall, A., Montalvo-Quirós, S. and Luque-Garcia, J. L. (2014). Isolation and identification of membrane vesicle-associated proteins in Gram-positive bacteria and mycobacteria. MethodsX 1: 124-129.
- McLoon, A. L., Guttenplan, S. B., Kearns, D. B., Kolter, R. and Losick, R. (2011). Tracing the domestication of a biofilm-forming bacterium. J Bacteriol 193(8): 2027-2034.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Brown, L., Kessler, A. and Casadevall, A. (2015). Vesicle Isolation from Bacillus subtilis Biofilm. Bio-protocol 5(5): e1409. DOI: 10.21769/BioProtoc.1409.
Category
Microbiology > Microbial biofilm > Biofilm culture
Microbiology > Microbial cell biology > Organelle isolation
Cell Biology > Organelle isolation > Outer membrane vesicles
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









