- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Synaptosomes from the Motor Cortex of Motor Skill Trained Mice
Published: Vol 5, Iss 4, Feb 20, 2015 DOI: 10.21769/BioProtoc.1398 Views: 14755
Reviewed by: Masahiro MoritaSoyun KimPamela Maher

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Immunopeptidomics Workflow for Isolation and LC-MS/MS Analysis of MHC Class I-Bound Peptides Under Hypoxic Conditions
Hala Estephan [...] Eleni Adamopoulou
Nov 20, 2025 1879 Views

Quantitative Proteomics of Nitrosylated Proteins in Melanoma Using the Biotin-Switch Technique Combined With Tandem Mass Tag Labeling
Vipin K. Yadav [...] Sanjay Premi
Dec 5, 2025 1549 Views

Optimized Secretome Sample Preparation From High Volume Cell Culture Media for LC–MS/MS Proteomic Analysis
Basil Baby Mattamana [...] Peter Allen Faull
Dec 20, 2025 1261 Views
Abstract
Learning and memory are thought to occur due to changes in synaptic strength. Strengthening of synapses due to Long Term Potentiation mechanisms are mediated by increases in synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) levels. Here we describe a protocol to isolate and quantify synaptic AMPAR subunit GluA1 levels from the motor cortex of mice which have undergone motor skill training.
Keywords: SynaptosomeMaterials and Reagents
- C57BL6 mouse (Mus musculus)
- Isoflurane (Isothesia) (Butler animal supplies)
- Sodium chloride (NaCl) (Thermo Fisher Scientific, catalog number: S-271 )
- Potassium chloride (KCl) (Thermo Fisher Scientific, catalog number: P217-500 )
- Sodium bicarbonate (NaHCO3) (Thermo Fisher Scientific, catalog number: S233-500 )
- Monosodium phosphate (NaH2PO4) (Thermo Fisher Scientific, catalog number: S369-500 )
- Magnesium sulphate heptahydrate (MgSO4.7H2O) (Thermo Fisher Scientific, catalog number: MG63-500 )
- Calcium chloride (CaCl2) (Thermo Fisher Scientific, catalog number: C79-500 )
- Dextrose (Thermo Fisher Scientific, catalog number: D16-500 )
- Sucrose (Thermo Fisher Scientific, catalog number: S5-3 )
- Radio immunoprecipitation assay (RIPA) lysis buffer (see Recipes)
- Roche complete protease inhibitor cocktail pellets (Roche Diagnostics, catalog number: 11697498 001 )
- Protease inhibitor (Sigma-Aldrich, catalog number: p8340 )
- Phosphatase inhibitor (Sigma-Aldrich, catalog number: 3 p0044 )
- Krazy glue
- Millipore anti GluA1 antibody(Millipore, catalog number: ab1504 )
- Cell signaling anti-rabbit HRP antibody (Cell Signaling Technology, catalog number: 7074S )
- Agar powder (Alfa Aesar, catalog number: A10752 )
- Artificial cerebrospinal fluid (ASCF) (see Recipes)
- High magnesium ACSF (see Recipes)
- Agar block (see Recipes)
- Sucrose media (see Recipes)
Equipment
- Leica vibratome S1000
- Scissors (one large and one small)
- Forceps
- Sharp blade
- Vibratome injector blade (Leica)
- 1.5 ml Eppendorf tube
- Pellet pestle motor hand held homogenizer (Kontes, catalog number: 749540-0000 )
- 4 ºC table top centrifuge
- Ruler
- Aerobic air mixture (Lindweld Alloy, model: MAA140 )
- Square petri dish with Grid (Thermo Fisher Scientific, catalog number: FB0875711A )
- Sonicator (Thermo Fisher Scientific)
Procedure
Note: Animals are trained on a relevant behavioral paradigm. For our experiments we used a motor forelimb reaching task (Padmashri et al., 2013). The technique described may be adopted to evaluate synaptic changes in any brain region relevant to a specific behavioral paradigm.
- Synaptosome preparation
- Prepare fresh artificial cerebrospinal fluid (1x ACSF) and bubble with aerobic air mixture at room temperature for at least 15 min.
- Prepare a 4 mM MgSO4 ACSF (high Mg2+ ACSF) and keep in an ice bath with constant bubbling.
- Anesthetize the animal using isoflurane gas and euthanize by decapitation.
- Make a sharp incision through the skin on top of the skull. Separate the skin from the bone.
- Make a straight incision from the Foramen Magna to the top of the skull. Separate out the two sections of the skull to expose the brain.
- Wash the brain with about 1 ml cold High Mg2+ ACSF.
- Isolate the brain and lay out transversely (Figure 1C). Cut off the cerebellum and the olfactory bulb (Figure 1D). Stand the brain on the caudal side and cut out the ventral portion of the brain (Figure 1E).
- Glue the caudal portion of the brain on the vibratome plate using a rectangle piece of agar as support (Figure 1F). Make sure the brain is submerged under ice cold High Mg2+ ACSF during slicing (Figure 1G). High Mg2+ ACSF is used as this prevents excitotoxicty to neurons by blocking N-methyl-D-aspartate receptor (NMDAR).
- Make thin coronal sections until the corpus callosum from the two hemispheres join (Figure 1H). Then make two 750 micrometer coronal sections which now contain the primary motor cortex M1 (1 mm anterior and 0.5 mm posterior to the bregma and 0.75 to 2.5 mm lateral).
- Incubate the slices in a submersion chamber in ACSF at room temperature for 1 h with constant bubbling.
- After incubation, place the brain slice on a petri dish under ASCF and constant bubbling at room temperature. Use a blade to isolate the forelimb region of primary motor cortex by making cuts 0.75 and 2.5 mm lateral to the midline (Figure 1I).
- Place the brain tissue into a 1.5 ml Eppendorf tube containing 300 µl of 0.32 M sucrose media 1 (see Recipes) containing protease inhibitor (1 tablet in 50 ml of media).
- Homogenize with a hand held homogenizer for one minute with up and down movements.
- Spin down the homogenized sample at 1,500 rpm for 10 min at 4 ºC. The pellet obtained contains nuclear and cellular debris and is discarded.
- Collect the supernatant which contains suspended synaptosomes and spin at 13,500 rpm at 4 °C for 20 min.
- The pellet obtained is the required synaptosomal preparation.
Note: The preparation obtained is an approximately 70% enriched preparation of synaptosomes. The preparation has contamination with mitochondria and debris from endoplasmic reticulum organelles. To prepare a more purified form of synaptosomes this preparation will have to be passed through a sucrose or percoll gradient and has not been described in this protocol.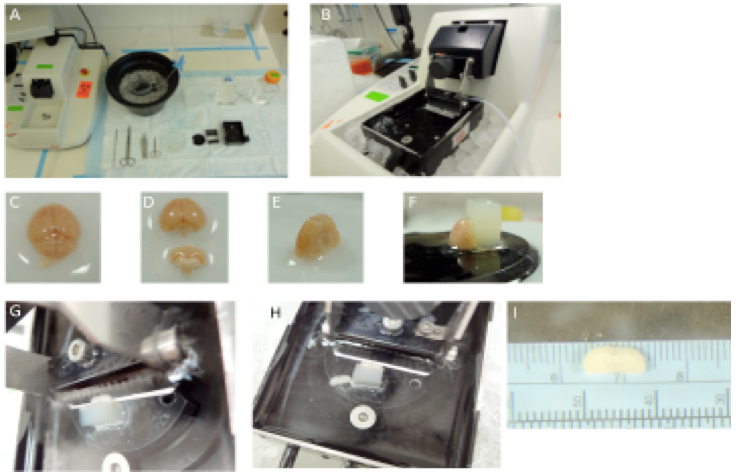
Figure 1. Preparation of brain slices on a vibratome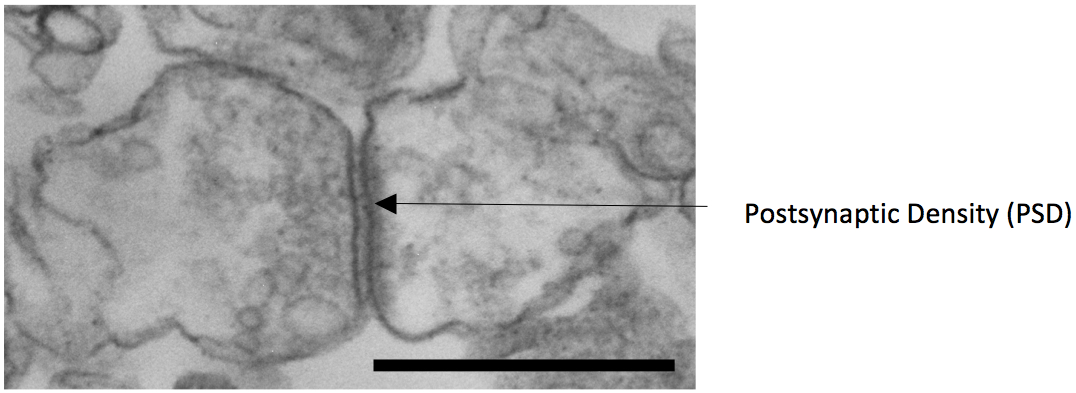
Figure 2. Electron microscopy image of a synaptosome with pre and postsynaptic component obtained from the protocol described (scale bar 1 micrometer)
- Prepare fresh artificial cerebrospinal fluid (1x ACSF) and bubble with aerobic air mixture at room temperature for at least 15 min.
- To evaluate synaptic GluA1 changes
- Aspirate out the supernatant from step A14.
- Re-suspend the pellet in 200 µl of RIPA lysis buffer.
- Lyse the preparation by sonication on ice (no post lysis spin required as the cellular debris is insignificant).
- Estimate the protein levels by relevant protein estimation protocols (BCA assay from Pierce).
- Run the protein lysates using a standard western blotting technique. We have run 20 microgram of protein for our experiments.
- To immunoblot use Millipore anti GluA1 at 1:1,500 dilution as primary antibody and Cell signaling anti-rabbit HRP as secondary antibody at 1:5,000 dilution.
- Aspirate out the supernatant from step A14.
Representative data
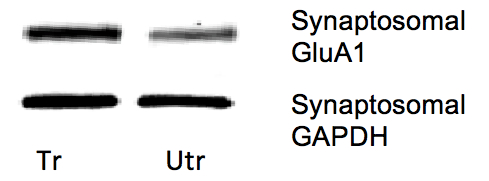
Figure 3. Representative western blots of synaptic GluA1 from forelimb M1 regions of trained (tr) and untrained (utr) hemispheres of motor skill trained mice. With motor skill training there is an increase in synaptic GluA1 in the forelimb region of the motor cortex following training. For quantification, GluA1 levels were normalized to GAPDH.
Note: We have evaluated other proteins such as mTOR, ERK, PSD-95, GLuA2, MAP2 from these preparations.
Recipes
- Artificial cerebrospinal fluid (ASCF)
Chemical Concentration (mM) g/L Sodium chloride (NaCl) 126 7.365 Potassium chloride (KCl) 3 0.2235 Monosodium phosphate (NaH2PO4) 1.25 0.1725 Sodium bicarbonate (NaHCO3) 26 2.185 Magnesium sulphate heptahydrate (MgSO4.7H2O) 1 0.120 Calcium chloride dihydrate
(CaCl2.2H2O)2 0.294 Dextrose 10 1.8 Water Make up to 1 L pH 7.4 Tonicity 300 mmoles/kg
Note: We prepare a 10x stock of ACSF excluding MgSO4, CaCl2 and dextrose and store at 4 °C. Before the experiment a 1x ACSF is prepared and Dextrose, magnesium and calcium salts are added. Both MgSO4 and CaCl2 are prepared and stored as 1 M stocks which are then added in required volume to the 1x ACSF. A point to note is that 1x ACSF is vigorously bubbled for at least 15 min prior to adding the CaCl2 to prevent calcium precipitation. Another alternative is to add the CaCl2 in small volumes with vigorous stirring on a stir plate. - High magnesium ACSF
Chemical ACSF 1x solution 1 L Magnesium sulphate heptahydrate (MgSO4.7H2O) 4 mM 0.984 g/L
Note: High Magnesium ACSF is used to block NMDA receptors channels and prevent cell death due to excitotoxicty. The Mg2+ is prepared as a 1 M stock and 0.15 ml of 1 M MgSO4 stock is added to 50 ml of previously prepared 1x ACSF to obtain a final concentration of 4 mM of Mg2+. - Agar block
Chemical Weight (in g) Agar powder 33.2 Sodium chloride 9 Water Make up to 1 L
Disperse the NaCl and Agar powder in water. Heat in a microwave oven until all the agar dissolves and a clear solution is obtained. Pour into a square petri dish and allow to cool to get agar blocks. Make small rectangles from the block and stick the breadth of the rectangle on the vibratome plate. - Sucrose media 1
Solution Volume 2.5 M sucrose 6.4 ml 0.5 M HEPES (pH 7.5) 500 µl 0.5 M EDTA (pH 8.0) 10 µl Water 43.09 ml
Sucrose media is stored at -20 °C. Roche Protease inhibitor cocktail tablet is added to the media before use (1 tablet in 50 ml). - RIPA lysis buffer
Ingredient Weight or volume Tris.HCl 0.3 g Sodium chloride 0.44 g Sodium dodecylsulphate 0.05 g Sodium deoxycholate 0.125 g Triton X 100 0.1 ml 0.5 M EDTA 0.05 ml Water Make up to 50 ml pH 7.4
Note: Lysis buffer is stored at room temperature. Before usage protease and phosphatase inhibitor are added (10 µl to 1 ml of lysis buffer). The lysis buffer is stored at 4 °C and used within 2 days of preparation.
Acknowledgments
This work was supported by a National Institute of Child Health and Human Development R01 HD67218 (to A. D.). We thank Dr. Gurudutt Pendyala for his valuable insights in establishing the protocol and Dr. Padmashri Ragunathan for comments on the manuscript. The protocol described is modified from previous publications (Weiler et al., 1981) to measure synaptic protein changes in discrete regions of the mouse cortex with behavior.
References
- Padmashri, R., Reiner, B. C., Suresh, A., Spartz, E. and Dunaevsky, A. (2013). Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome. J Neurosci 33(50): 19715-19723.
- Weiler, M. H., Gundersen, C. B. and Jenden, D. J. (1981). Choline uptake and acetylcholine synthesis in synaptosomes: investigations using two different labeled variants of choline. J Neurochem 36(5): 1802-1812.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Suresh, A. and Dunaevsky, A. (2015). Preparation of Synaptosomes from the Motor Cortex of Motor Skill Trained Mice. Bio-protocol 5(4): e1398. DOI: 10.21769/BioProtoc.1398.
-
Padmashri, R., Reiner, B. C., Suresh, A., Spartz, E. and Dunaevsky, A. (2013). Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome. J Neurosci 33(50): 19715-19723.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Biochemistry > Protein > Isolation and purification
Biochemistry > Protein > Quantification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link