- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Water Deficit Treatment and Measurement in Apple Trees
Published: Vol 5, Iss 3, Feb 5, 2015 DOI: 10.21769/BioProtoc.1388 Views: 10095
Reviewed by: Arsalan DaudiYuko KuritaYurong Xie

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Plate Growth Assay to Quantify Embryonic Root Development of Zea mays
Jason T. Roberts [...] David M. Braun
Oct 20, 2023 2256 Views

Detection and Quantification of Programmed Cell Death in Chlamydomonas reinhardtii: The Example of S-Nitrosoglutathione
Lou Lambert and Antoine Danon
Aug 5, 2024 1589 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1897 Views
Abstract
Water is considered perhaps the most limiting factor for plant growth and productivity (Boyer, 1982), and climate change predicts more frequent, more severe and longer drought periods for a significant portion of the world in coming years. Unfortunately, drought resistance is particularly difficult to measure due in part to the complexity of the underlying biology that contributes to a plant’s ability to cope with water limitations. For example, water deficit is frequently examined by detaching leaves or withholding water for a set period of time prior to tissue collection. Such approaches may elucidate the early stages of drought response but are generally not physiologically relevant for maintenance of drought resistance over a longer period. A more realistic approach is to impose a gradual water limitation with a sustained soil moisture level, particularly in the case of woody perennials. We describe here a protocol that imposes a long-term water deficit under controlled laboratory conditions that allow a molecular biology approach to understanding how woody plants survive severe water limitations. Representative data can be found in Artlip et al. (1997) and Bassett et al. (2014).
Keywords: Malus x domesticaMaterials and Reagents
- Woody plants (seedlings, grafted, or own-rooted)
- Potting soil (MetroMix 360; SunGro Horticulture)
- Water source for watering plants
- ‘Play’ sand (can be obtained from any ‘home and garden’ store) washed three times with hot tap water and dried
- Slow release fertilizer (Osmocote, Scott’s Miracle-Gro Products, catalog number: 19-6-12 N-P-K )
- MS salts (Phytotechnology Laboratories, catalog number: M524 )
- Sucrose (Phytotechnology Laboratories, catalog number: S391 )
- Agar (Fisher Scientific, catalog number: BP1423 )
- Myo-inositol (Sigma-Aldritch, catalog number: I3011 )
- IBA (Sigma-Aldritch, catalog number: I5386 )
- Nutrient solution (MiracleGro, Scott’s Miracle-Gro Products, catalog number: 24-8-16 N-P-K )
- Rooting medium (see Recipes)
Equipment
- 1 gallon plastic pots (or desired size to accommodate plants)
- Plastic beaker for measuring soil
- Aluminum foil (optional)
- Small ruler
- Oasis rooting cubes (Smithers-Oasis LC-1 Horticubes, catalog number: 5240 )
- Conviron TC16 tissue culture chamber (Conviron)
- Greenhouse or Growth chamber for maintaining appropriate light, temperature and photoperiod (equivalent to Conviron, models: PGV36 or PGW36 )
- Scale appropriate for weighing pots with plants, (e.g. Weigh-Tronix, model: 830 )
- Scholander pressure bomb (SoilMoisture Equipment Corp., model: 3005 Series )
Procedure
- Plants
- Own-rooted ‘Royal Gala’.
Explants were propagated in tissue culture as described by Norelli et al. (1988) and Ko et al. (2002), with root induction as described by Bolar et al. (1998). - Upon root formation (~ one month), the seedlings were transferred to Oasis rooting cubes, and maintained in a Conviron TC16 tissue culture chamber for one month (24 °C, 70% r. h., 20 h light, 70 μmol photons/m2/s PPFD), with watering as needed and nutrient solution application weekly per the manufacturer’s recommendation.
- Own-rooted ‘Royal Gala’.
- Growth conditions
- 5-L pots with equal volumes of Metromix 310 (horticultural vermiculite, Canadian Sphagnum peat moss, processed bark ash, composted pine bark) or equivalent product, and washed sand.
- The trees were grown in a glasshouse with supplemental lighting (High Pressure Sodium lamps) to maintain the day length at 16 h, and a maximum-minimum temperature range 35 °C to 20 °C. Trees were watered daily, with weekly application of nutrient solution, with supplemental application of Osmocote every two months at the indicated rate of 10 g.
- Age and size: Trees were in the glasshouse for a total of 8 months, with final stem diameter measurements ranging from 0.5 cm to slightly more than 1.0 cm, and heights varying from 1 to 2 m. It is preferable to select trees as close to the same size (stem diamter or height) as possible.
- 5-L pots with equal volumes of Metromix 310 (horticultural vermiculite, Canadian Sphagnum peat moss, processed bark ash, composted pine bark) or equivalent product, and washed sand.
- Water deficit
- Trees are placed in a Conviron PGV36 growth chamber (Conviron) at 25 °C day (16 h)/18 °C night (8 h) with light at 500 μmol photons/m2/s PPFD.
- The pot is then watered to saturation and weighed one to two hours later.
- To mimic field conditions where water is lost to the atmosphere by evapotranspiration (evaporation from the soil plus transpiration by the plant), the soil surface is left exposed. To measure transpiration alone, a moisture barrier, typically aluminum foil, is placed over the pot and around the stem of the tree.
- Water is withheld and the pot weighed at the same time of day on a daily basis. Control plants are watered to saturation (90-95% pot weight) every other day. Care should be taken not to over water the controls as this can cause ‘flooding’ symptoms, i.e., wilting, chlorosis etc.
- Water deficit should be imposed by withholding water until the pot masses are 45% of the saturated mass and maintained at this level for two weeks by adding back water to the 45% level; control trees are rewatered to the saturated weight. Sufficient trees should be used for statistical analysis (to be determined by the user). We typically used four replicates per apple line, per treatment and an equivalent number of control trees.
- Water status of plants should be determined in order to quantify the water deficit. Common measurements include water potential (Scholander pressure bomb with leaves, branches or stems; Scholander et al., 1964), leaf size (Bassett et al., 2011), leaf number (Figure 2) or relative water content (RWC; Sinclair and Ludlow, 1985), although Kramer (1990) argued that water potential is a preferable measure owing to its being commensurable with atmospheric or soil water measurements. This measurement should be done pre-dawn or with foil-covered leaves. A water status of -1.9 MPa can be achieved with peach trees (Artlip et al., 1997). Researchers in the field (e.g., Hsiao, 1973) consider a water potential of this magnitude to impose a severe stress on the plant.
- It is important to note that plant size relative to pot size is important (large plant in a small pot is not equivalent to a large plant in a large pot) to prevent plants from becoming root-bound. We found that 5 L pots were ideal to grow trees to 1 m prior to selection for the drought experiments. It should be emphsized that different cultivars or geographical populations can differ in their water use efficiency or dehydration tolerance. Obviously, if a lesser degree of stress is desired, more water added back would increase the water status of the tree.
- Trees are placed in a Conviron PGV36 growth chamber (Conviron) at 25 °C day (16 h)/18 °C night (8 h) with light at 500 μmol photons/m2/s PPFD.
Representative data
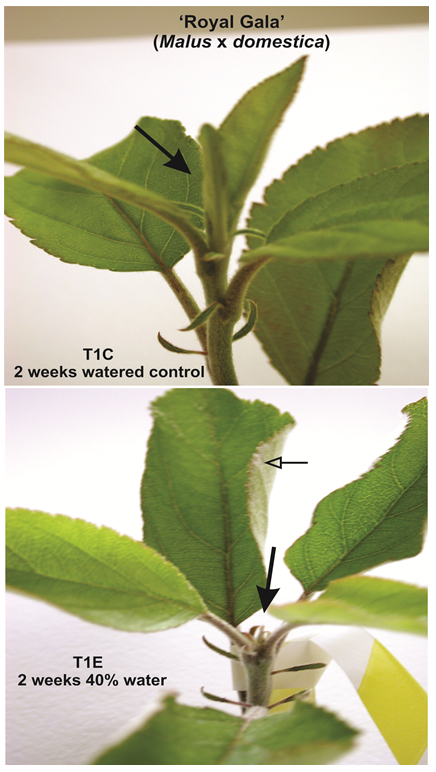
Figure 1. Young apple trees of cultivar Royal Gala from drought experiment. T1C: control trees two weeks after the beginning of the experiment. These trees were watered to saturation (90-95% pot weight). Arrow shows new leaf unrolling at shoot apex. T1E: experimental trees two weeks after being held at 40% saturation. Filled arrow marks dying shoot apex; open arrow shows leaf curling which is a common indicator of dehydration. Photographs were taken from Bassett et al. (2011) with permission from the American Society for Horticultural Science.
Figure 2. Typical water deficit experiment in the growth chambers
Apple trees ~ 1 m tall were selected, tagged and placed in the Conviron growth chamber. The figure shows trees with and without foil placed on the pots. Red tags represent water deficit treatment; blue tags represent well-watered controls. A. Water deficit treatment and controls without foil; B. Water deficit treatment with foil covering pot surfact.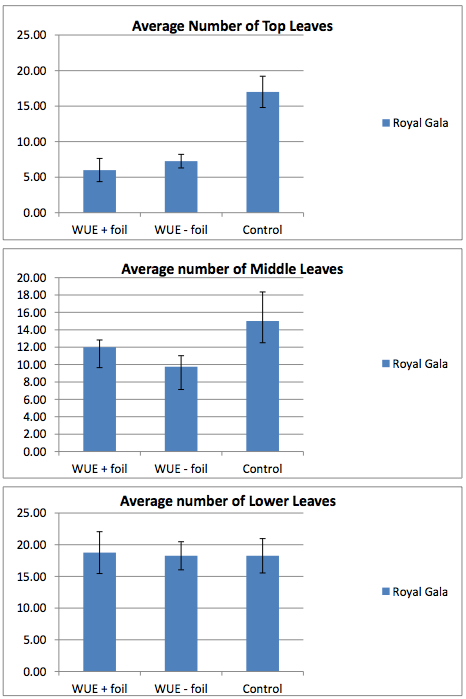
Figure 3. Leaf number of ‘Royal Gala’ trees subjected to a severe drought. The number of leaves along three regions of the stem were counted as follows: young leaves (1-2 cm long) at the top of the plant, leaves (~4-6 cm long) along the middle of the plant, and leaves (~7-8 cm long) along the lower portion of the plant. Pots of water-restricted plants were maintained with (WUE + foil) or without (WUE - foil) foil covers for the duration of the experiment. Controls were well watered with no foil on the pots. The number of leaves was counted every other day for two weeks. The greatest difference in leaf loss due to water deficit is seen in the youngest leaves just beginning expansion, whereas oldest leaves having expanded before the water deficit was imposed show the least difference.
Notes
- Most fruit trees are grafted onto a commercial rootstock. Use of grafted trees will reflect influences from both the scion and rootstock unless roots can be propagated from buds or stems of the desirable cultivar.
- ePure water is ≥ 18 Mohm-cm.
Recipes
- Rooting medium (pH 5.6)
- Ingredients per 1 L
ePure water 1 L MS Salts 2.15 g Thiamine-HCl (0.4 g/100 ml stock) 1 ml Myo-inositol (100 mg/ml stock) 1 ml Sucrose 20 g Agar 7 g Hormone IBA For root induction 2.5 mg/L For root elongation 0.0 mg/L - Preparation
Add 25-50% of water to a beaker on hotplate with constant stirring
Add MS salts and sucrose to beaker
Add agar and melt until completely dissolved
Add remaining water
Add Thiamine-HCl, Myo-inositol and IBA if needed
Calibrate pH meter and adjust pH to 5.6 with 1 M NaOH
Dispense into small baby food jars and autoclave for 8-10 min
- Ingredients per 1 L
Acknowledgments
This work was funded by USDA through the Agricultural Research Service as part of the in-house appropriated funding. We would like to acknowledge the contribution and excellent technical assistance of Sharon Jones.
References
- Artlip, T. and Wisniewski, M. (1997). Tissue-specific expression of a dehydrin gene in one-year-old Rio Oso Gem'Peach trees. J Amer Soc Hort Sci 122(6): 784-787.
- Bassett, C. L., Baldo, A. M., Moore, J. T., Jenkins, R. M., Soffe, D. S., Wisniewski, M. E., Norelli, J. L. and Farrell, R. E., Jr. (2014). Genes responding to water deficit in apple (Malus x domestica Borkh.) roots. BMC Plant Biol 14: 182.
- Bassett, C. L., Glenn, D. M., Forsline, P. L., Wisniewski, M. E. and Farrell, R. E. (2011). Characterizing water use efficiency and water deficit responses in apple (Malus× domestica Borkh. and Malus sieversii Ledeb.) M. Roem. HortScience 46(8): 1079-1084.
- Bolar, J. P., Norelli, J. L., Aldwinckle, H. S. and Hanke, V. (1998). An efficient method for rooting and acclimation of micropropagated apple cultivars. HortScience 33(7): 1251-1252.
- Boyer, J. S. (1982). Plant productivity and environment. Science 218(4571): 443-448.
- Hsiao, T. C. (1973). Plant responses to water stress. Annu Rev Plant Physiol 24(1): 519-570.
- Ko, K., Norelli, J. L., Reynoird, J.-P., Aldwinckle, H. S. and Brown, S. K. (2002). T4 lysozyme and attacin genes enhance resistance of transgenic 'Galaxy' apple against Erwinia amylovora. J Amer Soc Hort Sci 127(4): 515-519.
- Kramer, P. J. (1990). A brief history of water measurement in measurement techniques in plant science. In: Hashimoto, Y., Nonami, H., Kramer, P. J. and Strain, B. R. (eds). Academic Press, pp.45-68.
- Norelli, J., Aldwinckle, H. and Beer, S. (1988). Virulence of Erwinia amylovora strains to Malus sp. Novole plants grown in vitro and in the greenhouse. Phytopathology. 78:1292-1297.
- Scholander, P. F., Hammel, H. T., Hemmingsen, E. A. and Bradstreet, E. D. (1964). Hydrostatic pressure and osmotic potential in leaves of mangroves and some other plants. Proc Natl Acad Sci U S A 52(1): 119-125.
- Sinclair, T. and Ludlow, M. (1985). Who taught plants thermodynamics? The unfulfilled potential of plant water potential. Aust J Plant Physiol 12(3): 213-217.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bassett, C. L., Artlip, T. S. and Wisniewski, M. E. (2015). Water Deficit Treatment and Measurement in Apple Trees. Bio-protocol 5(3): e1388. DOI: 10.21769/BioProtoc.1388.
Category
Plant Science > Plant physiology > Abiotic stress
Plant Science > Plant physiology > Plant growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









