- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of CNS-infiltrating and Resident Microglial Cells
Published: Vol 5, Iss 2, Jan 20, 2015 DOI: 10.21769/BioProtoc.1385 Views: 16496
Reviewed by: Savita NairAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Time-Lapse Super-Resolution Imaging and Optical Manipulation of Growth Cones in Elongating Axons and Migrating Neurons
Masato Sawada [...] Kazunobu Sawamoto
Mar 20, 2025 2141 Views

Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
Jun 5, 2025 2620 Views

Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells
Hanki Kim [...] Jun Young Choi
Jun 20, 2025 1427 Views
Abstract
Variety of immunological and biochemical studies associated with infection or inflammation in the central nervous system (CNS) utilize CNS-resident and/or infiltrating cells which were isolated from the CNS of naïve and affected mice in order to investigate the underlying mechanisms and the potential roles of the cell populations. Mechanical and enzyme-based single cell preparations of CNS cells are subjected to a density gradient to obtain functional single cells. In combination with cell-specific biomarkers, the function and/or status of resident microglia and infiltrating lymphocytes, including B and T cells as well as macrophages, can be characterized.
Materials and Reagents
- Isoflurane (Vedco, catalog number: 50201 )
- Hanks' balanced salt solution (HBSS) (Cellgro, catalog number: 20-021-CV )
Note: 10x solution, diluted to 1x in house in sterile distilled water.
- RPMI-1640 medium (Sigma-Aldrich, catalog number: R8758 )
- Fetal bovine serum (FBS) (Atlanta Biologicals, catalog number: S11055H )
- Penicillin/streptomycin (Life Technologies, Gibco®, catalog number: 15140-122 )
- Collagenase type IV (Worthington Biochemical, catalog number: 4188 )
- DNase I (Sigma-Aldrich, catalog number: DN25 )
- Percoll (GE Healthcare, catalog number: 17-0891-01 )
- Phosphate buffered saline (PBS)
- Anti-CD45-allophycocyanin (APC) and anti-CD11b-phycoerythrin (PE) antibodies (both from BD, catalog numbers: 559864 and 557397 , respectively)
- Anti-CD8-APC (clone Ly-; 2) (BD, catalog number: 553035 )
- Anti-CD4-PE (clone L3T4) (BD, catalog number: 553049 ) antibodies
- Complete RPMI-1640 medium (see Recipes)
- Collagenase and DNase I solution (see Recipes)
- 100% Percoll (see Recipes)
- 70% Percoll (see Recipes)
- 30% Percoll (see Recipes)
Equipment
- Scissors and forceps
- 10 ml and 50 ml disposable plastic syringe (BD)
- 18 G needle (BD)
- Steel mesh (250 m) (VWR International, catalog number: AA13478-RR )
- 37 °C water bath
- Table top refrigerated centrifuge
- High-speed centrifuge (Beckman Coulter, model: J2-21 )
- 50 ml screw-cap tube
- LSRII Flow cytometer (BD)
- Low-speed table top centrifuge (1,000-3,000 rpm)
Procedure
- Naïve and virus (Theiler’s Murine Encephalomyelitis Virus)-infected mice (3-5 vs. 2-3, respectively) are anesthetized by Isoflurane at 7 days post infection.
- Anesthetized mice are transcardially perfused with 30 ml of sterile HBSS through left ventricle using 50 ml syringe.
- Decapitate the mice and excise the brain. Flush the spinal cord from the spinal column using the 10 ml syringe filled with HBSS.
- Excised brains and spinal cords are grinded separately or together using rubber-attached disposable syringe plunger through a steel mesh to prepare single-cells suspensions.
- Grinded brain and spinal cords are subjected to 300 μg/ml collagenase type IV plus 20 μg/ml and incubated at 37 °C for 45 min.
- Carefully decant the collagenase-containing supernatant after spinning the cell suspension at 630 x g (1,800 rpm) for 10 min at room temperature.
Isolation of CNS infiltrating cells using continuous gradient
A continuous 100% Percoll gradient is used to enrich CNS mononuclear cells. 10 ml of 100% Percoll is added to 20 ml of cell suspension in complete RPMI and then gently mixed by inverting the tube. Centifuge without braking, at 25,000 x g (15,000 rpm) and 37 °C for 30 min using a J2-21 centrifuge. Collect mononuclear cells in the bottom one-third by aspirating out the cell debries in the top 15-20 ml.
- Transfer the cells in the bottom Percoll gradient (10-15 ml) to v-bottom 50 ml tube.
- Bring up the volume to 50ml with HBSS or RMPI, gently mix and then centrifuge at 630 x g (1,800 rpm) for 10 min.
- Resuspend cells in complete RPMI medium.
- Approximately 1-2 x 106 cells are obtained from the brain and spinal cord per mouse at 7 days post TMEV infection. Approximately 2-5 x 105 cells are obtained from the CNS of a naïve mouse.
Isolation of CNS infiltrating cells using discontinuous gradient
Alternatively, microglia and infiltrating mononuclear cells are isolated at the interface of 70% and 30% Percoll. In particular, microglia from naïve mice are isolated using this more defined method. Single-cell suspensions of 3-5 brains and spinal cords from naïve mice or 2-3 brains and spinal cords from infected mice, which are prepared as described above (steps 1-6), are subjected to a discontinuous Percoll gradient (70 and 30%) at 2,800 x g (5,000 rpm) and 20 °C for 20 min (resuspend cells in 15 ml 30% percoll and underlay 20 ml 70% Percoll). The cells that accumulated in the interface of the gradient are collected and transferred to v-bottom 50 ml tube to wash.
- Isolated CNS-infiltrating mononuclear cells (5 x 105) are stained and analyzed by flow cytometry after gating live cells based on FSC and SSC (see Notes). The expression of CD45 and CD11b was measured using anti-CD45-allophycocyanin (APC) and anti-CD11b-phycoerythrin (PE) antibodies to distinguish CNS-resident CD45int microglia from infiltrating CD45hi leukocytes, in conjunction with their expression of CD11b (macrophages) or lack of it (lymphocytes). Specific cell populations such as B cells, CD4 and CD8 T cells are also either isolated or characterized in conjunction with flow cytometry using cell-specific markers.
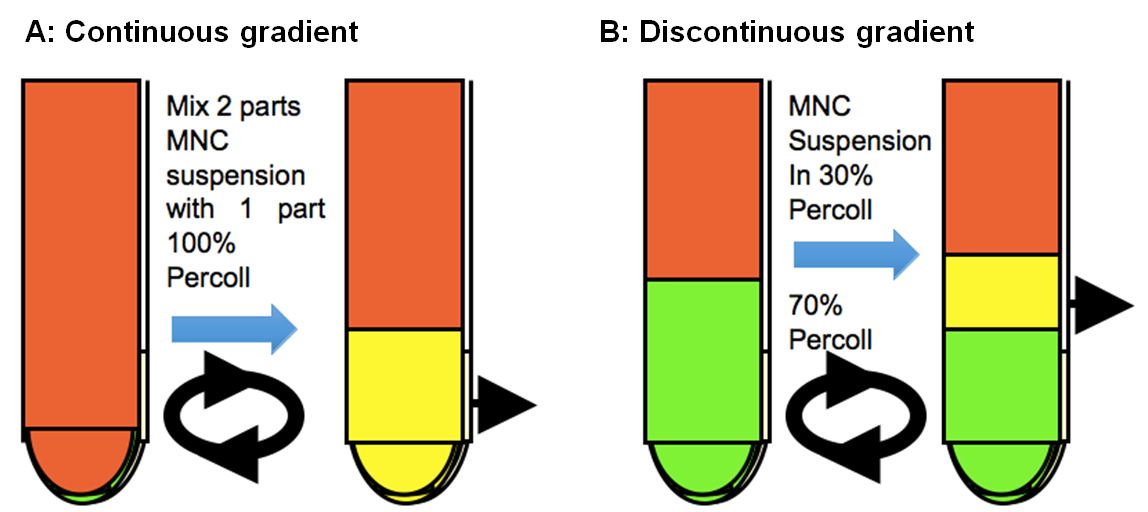
Figure 1. Continuous and discontinuous Percoll gradient centrifugations
Representative data
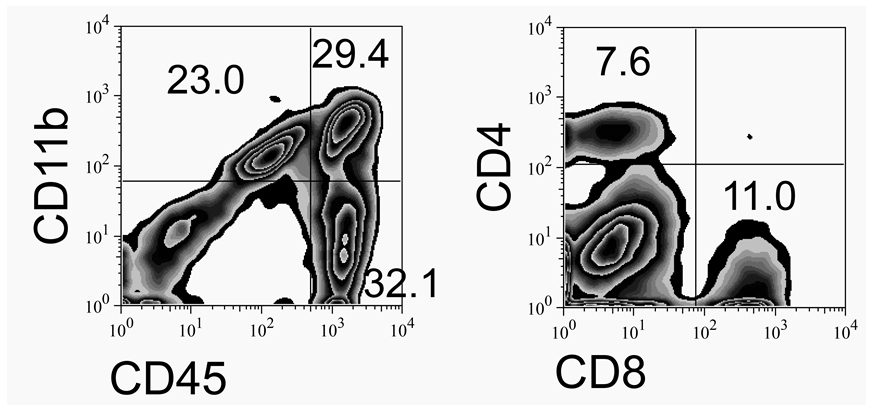
Figure 2. A representative result from continuous Percoll gradient of TMEV-infected CNS cells
Flow cytometric analysis of CNS infiltrating cells isolated using continuous Percoll gradient at 7 days post infection is shown.
Notes
- A brief flow cytometry method is described below.
- After isolation of cells, Fc receptors were blocked using 50 μl of 2.4G2 hybridoma (ATCC) supernatant by incubation at 4 °C for 30 min.
- Cells were then stained with allophycocyanin-conjugated anti-CD8 (clone Ly-2) and PE-conjugated anti-CD4 (clone L3T4) at 4 °C for 30 min.
- To visualize macrophage and microglial populations, cells were stained with PE-conjugated anti-CD11b (clone M1/70) and allophycocyanin-conjugated anti-CD45 (clone Ly-5) antibodies. Antibodies diluted in Facs buffer (1% FBS, 0.09% sodium azide in PBS). After incubation, cells were washed with FACS buffer and resuspend the cells in 400 μl of FACS buffer.
- After isolation of cells, Fc receptors were blocked using 50 μl of 2.4G2 hybridoma (ATCC) supernatant by incubation at 4 °C for 30 min.
Recipes
- Complete RPMI-1640 medium (100 ml)
9 parts of RPMI and 1 part of fetal bovine serum
Add 1 ml of 100x penicillin/streptomycin
- Collagenase and DNase I solution (100 ml)
Mix 0.03 g Collagenase IV and 200 μl (10 mg/ml in PBS) DNase I into 100 ml HBSS
Filter (0.22 μm) sterilize the solution
These solutions are prepared fresh and kept at 4 °C for each use
- 100% Percoll (10 ml)
Mix 1 ml 10x PBS and 9 ml Percoll
- 70% Percoll (100 ml)
Mix 70 ml 100% Percoll and 30 ml PBS
- 30% Percoll
Mix 30 ml 100% Percoll and 70 ml PBS
Acknowledgments
This protocol was adopted from many previous studies including the referenced work below and was supported by NIH (2RO1 NS28752 and NS33008) and NMSS (RG 4001A6 and RG 4342A7) grants.
References
- Havenith, C. E., Askew, D. and Walker, W. S. (1998). Mouse resident microglia: isolation and characterization of immunoregulatory properties with naive CD4+ and CD8+ T-cells. Glia 22(4): 348-359.
- Hou, W., Jin, Y. H., Kang, H. S. and Kim, B. S. (2014). Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. J Virol 88(15): 8479-8489.
- Jin, Y. H., Mohindru, M., Kang, M. H., Fuller, A. C., Kang, B., Gallo, D. and Kim, B. S. (2007). Differential virus replication, cytokine production, and antigen-presenting function by microglia from susceptible and resistant mice infected with Theiler's virus. J Virol 81(21): 11690-11702.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jin, Y. and Kim, B. S. (2015). Isolation of CNS-infiltrating and Resident Microglial Cells. Bio-protocol 5(2): e1385. DOI: 10.21769/BioProtoc.1385.
Category
Immunology > Immune cell isolation > Lymphocyte
Microbiology > Microbe-host interactions > Virus
Neuroscience > Cellular mechanisms > Cell isolation and culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









