- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Migration Assays for Neural Stem Cells, Intermediate Neurogenic Progenitors and Immature Neurons
Published: Vol 5, Iss 1, Jan 5, 2015 DOI: 10.21769/BioProtoc.1371 Views: 18919
Reviewed by: Xuecai GeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Time-Lapse Super-Resolution Imaging and Optical Manipulation of Growth Cones in Elongating Axons and Migrating Neurons
Masato Sawada [...] Kazunobu Sawamoto
Mar 20, 2025 2139 Views

Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells
Hanki Kim [...] Jun Young Choi
Jun 20, 2025 1426 Views

Utilizing EdU to Track Leukocyte Recruitment to the Brain
Zoie K. Lipfert [...] David P. Sullivan
Dec 5, 2025 1564 Views
Abstract
In the vertebrate central nervous system (CNS), different neural precursor populations such as neural stem cells (NSCs), intermediate neurogenic progenitors (INPs) and immature neurons have to migrate from their places of birth to their location of function. Coordinated migration is mediated by direct cell-cell interactions and by extracellular matrix components, chemoattractants as well as repellents. The migration potential of such populations as well as the responsiveness to chemoattractive compounds can be addressed in isolated cells using in vitro migration assays. Here we describe two migration assays, a matrigel migration assay and a Boyden chamber migration assay, which allow the in vitro assessment of neural migration under defined conditions (Ladewig, Koch and Brüstle, 2014). A matrigel matrix is a soluble basement membrane extract. The major components of matrigel matrix are collagens, laminin and proteoglycans, which provide the substrate for migrating cells. In the matrigel assay migration can be analyzed using a phase contrast microscope. The Boyden chamber assay (Richards and McCullough, 1984) is based on microchemotaxis chambers, which consist of two compartments separated by a membrane with a defined pore size. Cells can be plated in the upper compartment and allowed to migrate through the pores towards the lower compartment, in which a potential chemotactic agent is loaded. Cell migration can be analyzed following fixing and immunohistochemical staining. In principle, the described protocols should be applicable to other cell populations such as endothelial cells or cancer cells using conditions adapted to the individual needs of the specific cell type.
Materials and Reagents
- Neural cell populations: e.g. pluripotent stem cell (PSC) derived neuroepithelial stem cells (lt-NES) and neurons derived thereof (Ladewig et al., 2008; Koch et al., 2009)
- DMEM/F12 (high glucose) (Life Technologies, catalog number: 11320074 )
- Neurobasal medium (Life Technologies, catalog number: 21103049 )
- B27 supplement (Life Technologies, catalog number: 17504044 )
- N2 supplement (GE Healthcare, catalog number: T1129,2205 )
- Glucose (Roche Diagnostics, catalog number: HN06.3 )
- Trypsin/EDTA (Life Technologies, catalog number: 15400054 )
- Trypsin inhibitor (Life Technologies, catalog number: 17075029 )
- DNAse (CellSystems Biotechnologie Vertrieb GmbH, catalog number: LS002140 )
- Specific for matrigel migration assay
- Specific for Boyden chamber migration assay
- Millicell culture plate inserts 8-μm pore size (Millipore, catalog number: PI8P01250 )
- Nylon mesh (Pall, http://www.pall.com)
- 24 well plate (VWR International, catalog number: 734-0056 )
- Poly-l-ornithine (Sigma-Aldrich, catalog number: P3655-1G )
- Laminin (Life Technologies, catalog number: 23017015 )
- PBS (Life Technologies, catalog number: 14190094 )
- Chemoattractant such as FGF2 (R&D Systems, catalog number: 233-FB/CF ) or VEGF (R&D Systems, catalog number: 236-EG-01M )
- Potential inhibitors for used chem¬oattractant such as VEGF receptor 1 and VEGF receptor 2 blocking antibodies (both R&D Systems, catalog numbers: AF321 and AF357 ) and the FGF2 neutralizing antibody (Millipore, clone bFM-1)
- Cotton bud (drug store, e.g. Q-tip®)
- Solutions and antibodies for standard immunohistochemical staining
- Millicell culture plate inserts 8-μm pore size (Millipore, catalog number: PI8P01250 )
- Neural stem cell media (see Recipes)
- Neuronal differentiation media (see Recipes)
Equipment
- Cell culture centrifuge (e.g., Megafuge 1.0 R, Kendro Laboratory)
- 37 °C, 5% CO2 cell culture incubator (e.g., Thermo Fisher Scientific, model: Heracell 240 )
- Counting chamber (e.g., Fuchs-Rosenthal, Marienfeld)
- Glass object slide (e.g., Thermo Fisher Scientific, Superfrost Plus)
- Microscope (e.g., ZEISS, model: Axiovert 200M )
Procedure
- Matrigel migration assay
- Thaw matrigel matrix at 4 °C on ice overnight.
- Dilute matrigel matrix at a ratio of 1:2 in cold DMEM/F12.
Note: 1 ml of matrigel will be sufficient to prepare 12 wells of a 4 well dish.
- Add 250 μl of the matrigel matrix mixture per well of a 4-well tissue culture dish and incubate in the cell culture incubator (at 37 °C) for at least 30 min for hardening.
Note: Store pipet tips at -20 °C before using.
- Trypsinize cells and count them using a counting chamber.
Note: At this step cell suspension can be incubated with 0.1% DNAse to avoid cell clumping.
- Pellet cells by centrifugation at 300 x g for 4 min.
- Remove supernatant and resuspend 100,000 cells/µl in cell culture media (e.g. Neuronal media containing DMEM/F12 with one vol% N2 supplement and Neurobasal with two vol% B27 supplement mixed at a 1:1 ratio).
Optional: Add 5 mM Rock inhibitor for cell survival.
- Spot 1 µl of the cell suspension in the middle of the dish on the gel surface.
Note: Specified 4 well dishes have a very slight round bottom which supports precise spotting.
- Incubate dishes for 10 min by carefully transferring them to the cell culture incubator until cells are attached.
- Carefully cover the cells with respective cell culture media and cultivate them in the cell culture incubator until analysis.
- Radial migration from the center of the cell clumps can be analyzed at different time points using static or live cell microscopy (see Figure 1).
- Thaw matrigel matrix at 4 °C on ice overnight.
- Boyden chamber migration assay
- Place Millicell culture plate inserts into 24 well plates and coat them from both sides with poly-l-ornithine. Incubate the culture plate for at least 2 h at 37 °C (e.g. in the cell culture incubator).
- Wash the 24 well dish with the millicell culture plate inserts 3 times with PBS followed by coating with laminin (1:1,000 diluted in PBS).
- Wrap the dish with parafilm and store at least over night at 4 °C.
Note: Experiments should always be performed in triplicates.
- Trypsinize cells and count them using a counting chamber.
Note: At this step cell suspension can be incubated with DNAse to avoid cell clumping and filtered through a nylon mesh (before counting).
- Pellet the cells at 300 x g for 4 min.
- Remove the supernatant and resuspend 1 x 106 cells/ml in neural differentiation media.
Note: The media used should not contain any growth factors or cytokines.
- Remove the coating suspension and load the bottom well of the chamber with 400-450 µl media with or without the chemoattractant of interest (e.g. 10 ng/ml VEGF or 10 ng/ml FGF2).
Notes:
- It is crucial that the media in the bottom well is covering the bottom side of the membrane but should not rise to the upper side.
- To avoid bubbles, carfully tip the pipette to the fringe of the chamber.
- Use respective chemoattractive-blocking agents as control (e.g. VEGF receptor 1 and VEGF receptor 2 blocking antibodies and FGF2 neutralizing antibody).
- It is crucial that the media in the bottom well is covering the bottom side of the membrane but should not rise to the upper side.
- Plate 100 µl of the cell suspension on the poly-l-ornithine/laminin coated membrane that separates the upper and the lower well.
Note: Do not touch the filter and avoid creating bubbles.
- Place the chamber in the 37 °C, 5% CO2 incubator for 6-20 h. The incubation time varies considerably depending on the cell type and chemotactic factor.
- Fix the dish (with the millicell culture plate insert) with 4% PFA and wash twice with PBS.
- Stain the upper and lower side of the membrane with DAPI.
Note: Check under the fluorecence microscope whether cell destribution is uniform (on both the upper and lower side of the membrane) and proceed only with those membranes. Non-uniform distribution of cells is most likely due to incomplete coating of the membrane. Take care that the membrane is not getting dry during the coating (point 1-3).
- Wipe off cells on the upper side of the membrane (non-migrated cells) with a cotton bud. Repeat this step at least twice.
- Stain the millicell culture plate inserts (in the 24 well dish) for appropiate markers (e.g. ßIII tubulin to identify neurons, nestin for neural progenitors).
- Cells reaching the bottom side of the membrane can be quantified under the flurecence microscope (Figure 2).
Note: The millicell culture plate inserts can be placed on a glass object slide, use a drop of PBS to keep the membrane wet.
- Place Millicell culture plate inserts into 24 well plates and coat them from both sides with poly-l-ornithine. Incubate the culture plate for at least 2 h at 37 °C (e.g. in the cell culture incubator).
Representative data
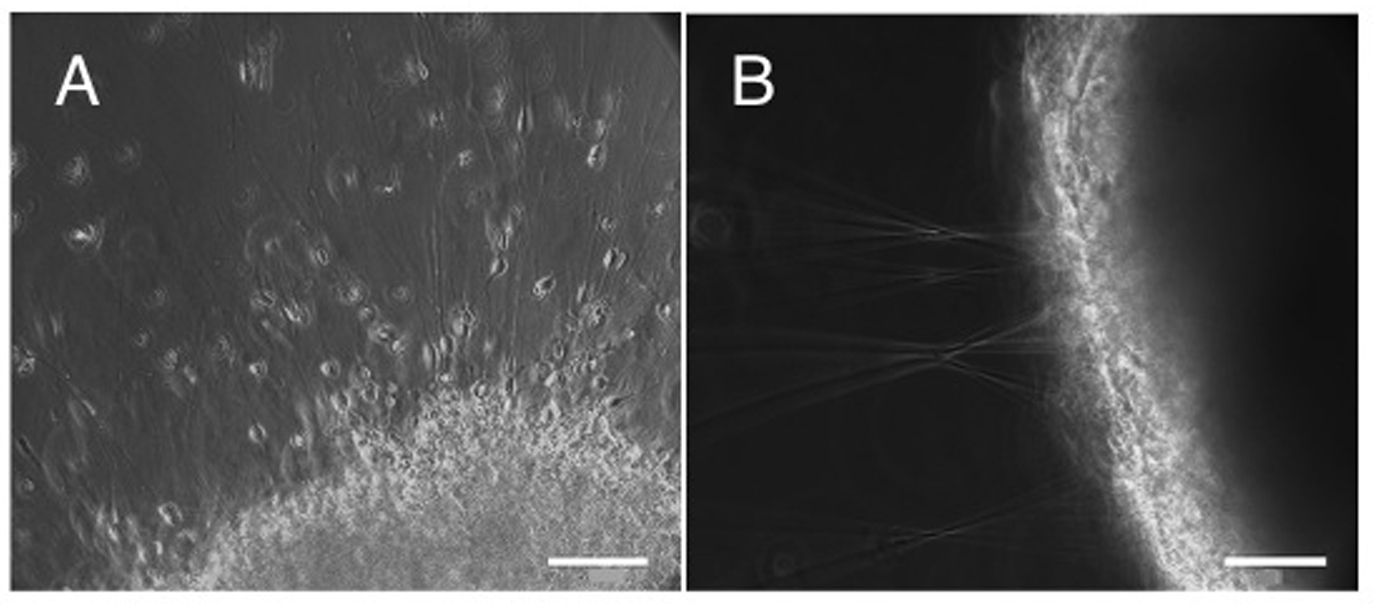
Figure 1. (A-B) Matrigel migration assay addressing the migration capacity of lt-NES cell derived purified neurons in comparison to neurons within non-purified population. Equal numbers of cells were plated on matrigel matrix and analyzed 48 h later. Purified neurons showed a radial symmetric distribution across a large area with migration of individual neurons from the plating site A. In contrast, the non-purified population (containing neurons as well as progenitor cells) formed spherical clusters with hardly any neurons leaving these aggregates. Instead, radial axonal outgrowth was observed B. Scale bar: A-B. 200 µm
A
B
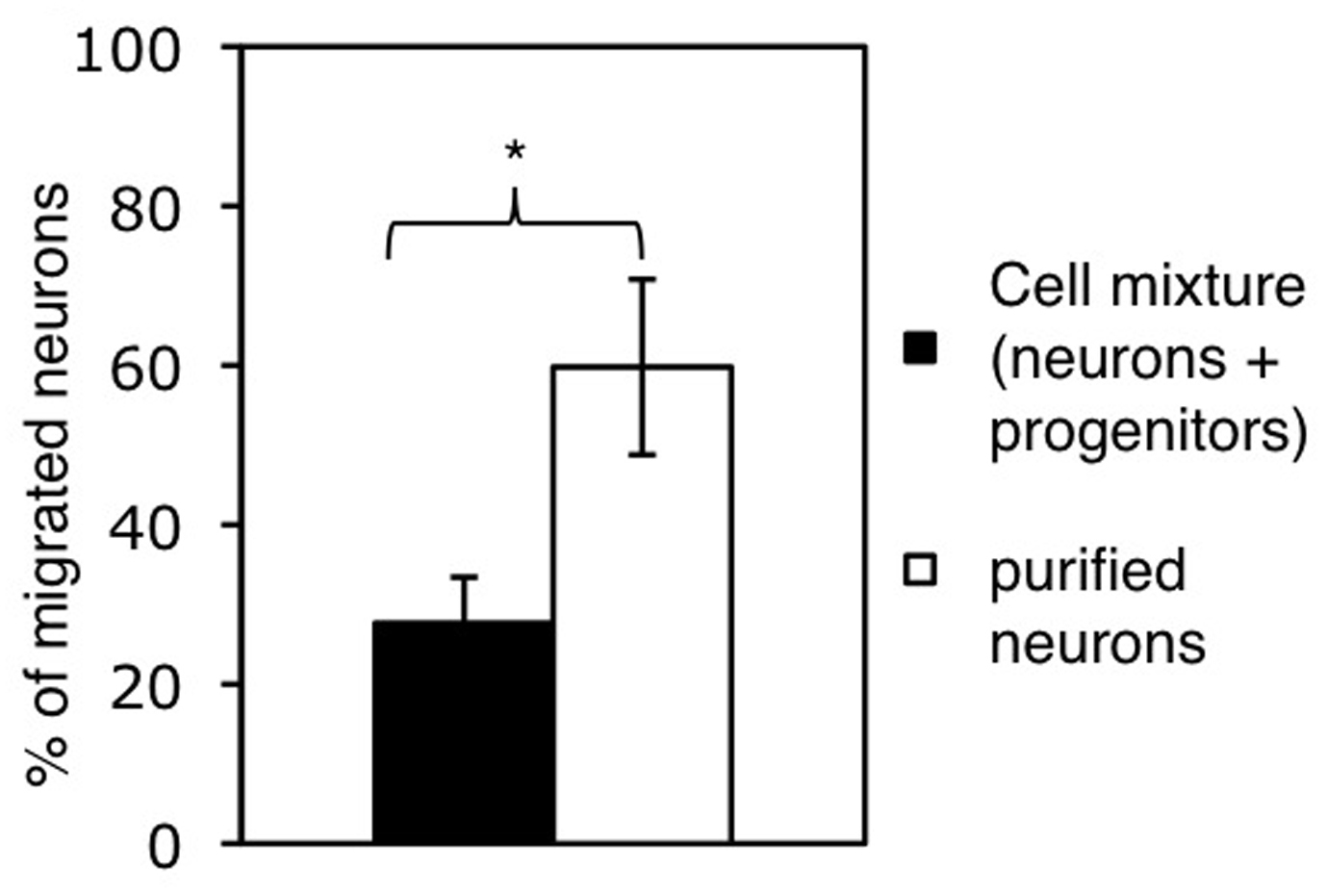
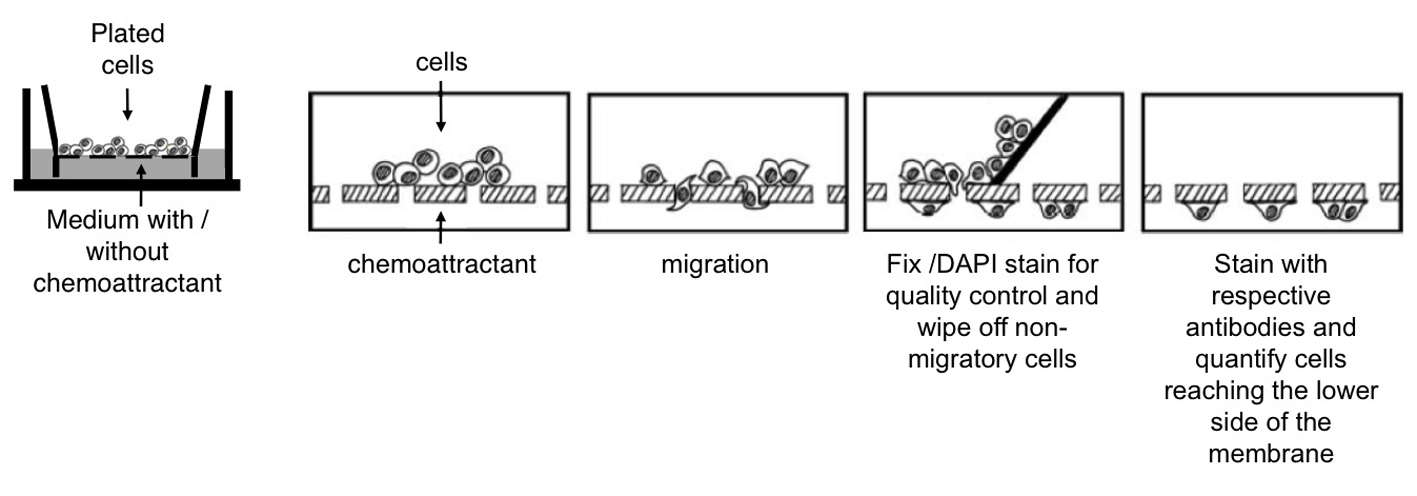
Figure 2. Cell migration studied by using millicell culture plate inserts. A. Cells are plated on the membrane of the upper well. Chemoattractants can be added to the lower well. Migration of cells from the upper well through the membrane can be measured by wiping off the remaining cells from the upper side of the membrane using a cotton bud and counting the cells that reached the bottom side. Adapted from Erlandsson (2003). B. Assessing the migration of neurons within a cell mixture (containing neurons and progenitor cells) and of purified neurons. Bars represent the percentage of neurons reaching the lower side of the membrane after 20 h. Cell numbers were normalized to the number of neurons plated and shown as mean+SD (*P < 0.05).
Notes
The quality of the Millicell culture plate inserts can vary, thus it is crucial to ensure that the cell destribution is uniform on both the upper and lower side of the membrane as described in point 11 of the Boyden chamber migration protocol.
Recipes
- Neural stem cell media
500 ml DMEM/F12, high glucose
5 ml N2 supplement
1.68mg glucose
- Neuronal differentiation media
50% neural stem cell media
48% neurobasal media
2% B27 supplement
Acknowledgments
This work was supported by the European Union (grant 222943 Neurostemcell; LSHG-CT-2006-018739, ESTOOLS), the German Research Foundation (DFG; SFB-TR3), the Hertie Foundation and the Ministry of Innovation Science and Research of North Rhine-Westphalia (Junior Research Group, L-072.0081).
References
- Erlandsson, A. (2003). Neural Stem Cell Differentiation and Migration.
- Koch, P., Opitz, T., Steinbeck, J. A., Ladewig, J. and Brüstle, O. (2009). A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci U S A 106(9): 3225-3230.
- Ladewig, J., Koch, P. and Brüstle, O. (2014). Auto-attraction of neural precursors and their neuronal progeny impairs neuronal migration. Nat Neurosci 17(1): 24-26.
- Ladewig, J., Koch, P., Endl, E., Meiners, B., Opitz, T., Couillard-Despres, S., Aigner, L. and Brüstle, O. (2008). Lineage selection of functional and cryopreservable human embryonic stem cell-derived neurons. Stem Cells 26(7): 1705-1712.
- Richards, K. L. and McCullough, J. (1984). A modified microchamber method for chemotaxis and chemokinesis. Immunol Commun 13(1): 49-62.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ladewig, J., Koch, P. and Brüstle, O. (2015). In vitro Migration Assays for Neural Stem Cells, Intermediate Neurogenic Progenitors and Immature Neurons. Bio-protocol 5(1): e1371. DOI: 10.21769/BioProtoc.1371.
Category
Neuroscience > Cellular mechanisms > Cell isolation and culture
Developmental Biology > Cell growth and fate > Neuron
Cell Biology > Cell movement > Cell migration
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









